How do I make my views known?
NHS England and the National Institute for Health and Care Excellence (NICE) welcome your comments on our proposals for an integrated, rules-based medical technology (medtech) pathway. This public engagement is open for 12 weeks, from Thursday 23 May 2024 until midnight on Thursday 15 August 2024.
Responses can only be submitted via the online feedback form on the NHS England engagement portal. If you require any of these documents in an alternative format, please contact england.medtechpathwayqueries@nhs.net
We are eager to hear from industry stakeholders, patient groups, carers, NHS trusts and integrated care boards as well as the wider public to inform our proposals for the development of this pathway.
Your responses will be public documents and all, or any part, of a response may be publicly available. If you wish to refer to confidential information in your response, please provide it in a separate document, clearly mark each page ‘confidential’ and send to england.medtechpathwayqueries@nhs.net
NHS England and NICE are subject to the Freedom of Information Act. While both organisations respect the confidentiality of any information provided to them, you should be aware that we may be obliged to release even confidential information under that Act. Please do not include sensitive personal data in your response.
Post engagement
NHS England, NICE, and the Department for Health and Social Care (DHSC) will consider all relevant feedback. NHS England and NICE are expecting many responses and will publish our feedback as a report on the NHS England website, capturing all material issues raised and sharing the insights and our learnings from responses.
Anyone responding to this engagement should note that responses may be published in full as part of NHS England and NICE’s commitment to openness and transparency.
Engagement questions
Engagement questions are included throughout in the relevant sections, and in the appendix. We look forward to receiving your responses.
Foreword
Medical technology (medtech) touches the lives of every person in the UK. From ultrasound scans before we are born and wearable devices that help us live healthier lives, to products that are implanted into our bodies and cutting-edge genomic testing and sequencing. What we consider to be medtech covers a wide range of products, devices, digital technologies and diagnostics.
Medtech not only plays a vital role in the nation’s health, transforming clinical pathways and the lives of patients, but is also a keystone of the British economy. With an annual turnover of £34 billion, over 4,400 UK businesses make up this thriving sector that supports 154,000 UK jobs; when this industry succeeds it is not only NHS patients that benefit, but also our local communities. That is why this consultation on the establishment of an integrated, rules-based pathway for medtech matters for everyone.
In February 2023, DHSC published a new Medtech strategy, setting out a roadmap of how to make sure the health and social care system over the next 5 to 10 years fully benefits from safe, effective and cutting-edge medtech innovations. One year into delivery of this strategy, DHSC recently updated system partners on progress so far.
NICE, NHS England and DHSC are determined to work in partnership to create the right conditions for innovators to thrive, with the health service continuing to act as powerful driver of innovation.
But there are several challenges that need to be overcome to ensure NHS patients get the right product, at the right price, in the right place. Currently there are multiple pathways supporting the introduction of medtech into the NHS but until now we’ve not had clear and consistent rules that determine how medical technologies should be assessed by NICE or commissioned by the NHS. The category of medtech is incredibly broad and varied, and there is often limited clinical evidence to assess clinical and cost effectiveness. As the pace of innovation accelerates, there is now an opportunity to provide greater coordination for new and existing products, and clarity on what to expect from a NICE evaluation and the role the NHS plays.
NHS England and NICE, in conjunction with DHSC, have worked together to develop a proposed pathway that brings together the entire medtech lifecycle – from early-stage technologies where additional evidence generation is needed, to groups of new, cutting-edge technologies ready for a NICE assessment of clinical and cost effectiveness, to existing technologies already in use in the NHS to drive greater value. The consultation sets out two key elements to the proposed pathway.
Firstly, it outlines the ‘rules’ and principles on which NICE and NHS England will work in partnership to evaluate, commission and fund medical technologies. The proposals set out the key phases of an integrated NICE and NHS pathway for medtech devices, digital technologies and diagnostics that will apply across a product’s lifecycle.
Secondly, the proposals outline how the NHS will support the routine commissioning of technologies determined to be clinically and cost effective by NICE in the NHS.
Whilst we expect the number of topics that can be considered through the pathway to grow over time, we recognise that it would not be appropriate to appraise all new medtech products coming to market given the scale and pace of innovation, so prioritisation based on those technologies that have the greatest potential to improve healthcare and support NHS priorities will continue to be important. This pathway will be one key mechanism to bring innovative products to market at scale, working cohesively with a wider suite of initiatives to improve adoption.
We recognise that NICE’s methodologies and processes are evolving, and this will be considered as we shape the development of the pathway. In addition, public, patient and clinician involvement will be vital to developing a pathway fit for the future.
We will use this consultation to engage with people across industry, the NHS – including clinicians and those who use health services – to understand their views on what they need from an integrated, rules-based pathway for medtech; and in doing so we will refine, evolve and adapt our proposals and how they are implemented. We thank you in advance for taking the time to contribute your ideas.
1. Background and purpose
1.1 This document sets out proposals for moving towards a more integrated, rules-based, and predictable pathway for the evaluation, funding, and commissioning of medical technology (medtech) in the NHS. The intention is that the pathway will apply across the entire lifecycle from promising early-stage technologies; to groups of new, innovative products ready for a National Institute for Health and Care Excellence (NICE) assessment of clinical and cost-effectiveness; as well as existing technologies in widespread use where there is scope to drive greater value. By establishing the pathway, NHS England and NICE are seeking to improve outcomes for patients, provide greater certainty for medtech innovators and suppliers, and drive better value for money for taxpayers and the NHS.
1.2 Medtech innovation is a broad term with a potential scope covering a multitude of digital, diagnostic, and medical device sectors. The scale and diversity of each of those sectors can also be vast. To illustrate, there are an estimated 2 million different kinds of medical devices on the world market, categorised into more than 7000 generic devices groups.
1.3 NHS patients benefit enormously from innovative and cutting-edge technologies that are used to diagnose, monitor, and deliver treatment. These range from coronary angioplasty, to restoring vision through artificial corneas, to digital technology that can help people manage their conditions, to devices that enable surgical interventions like mechanical thrombectomy for stroke and advanced robotics. These technologies have the potential to offer huge system benefits to the NHS and its patients, by transforming clinical practice and improving NHS productivity, especially given exciting recent advances in artificial intelligence-enabled imaging and genomic testing.
1.4 In February 2023, DHSC published a new Medtech strategy. This set out how, over the next 5 to 10 years, the health and social care system can reliably access safe, effective, and innovative technologies. One year into the delivery of the Medtech strategy, DHSC has published an update on progress towards streamlining the innovation pathway, essential to ensure patients have access to the best technology and the NHS keeps up with the fast pace of technological advancement.
1.5 As the scale and pace of innovation increases, it is more important than ever that the NHS and NICE provide a clearer, more predictable, and more rules-based approach to access for medtech. This will ensure the health service – nationally and at an integrated care board level – is using technologies recommended by NICE that deliver the most health gain in a way which drives value for money and encourages clinical adoption.
1.6 NICE provide a robust, internationally recognised gold standard in evidence-based guidance to the health service. The pathway will apply NICE’s methods and processes to devices, diagnostics, and digital technologies that: deliver treatment – like those implanted during surgical procedures; give greater independence to patients to manage and control their condition; and detect or monitor medical conditions.
1.7 NICE will determine the clinical and cost-effectiveness of the most promising new medical technologies so that NHS patients benefit from high-quality, up-to-date guidance and advice. For those technologies that are recommended by NICE as clinically and cost-effective and that meet an NHS affordability test, there will be a commitment to automatic identification of funding to support routine commissioning, providing a clear incentive for industry to continue to invest in evidence generation and partnership with the health service and NICE.
1.8 NICE is already updating its evaluation methods and processes so it can respond to emerging innovation across diagnostics, devices, and digital areas. Alongside this, the Medicines and Healthcare products Regulatory Agency has developed a roadmap to evolve its own regulatory processes for medtech. The proposals in this document will build on and work alongside these initiatives.
1.9 The pathway will evolve over time, including learning lessons from current and past programmes and initiatives. NICE and NHS England will start selective and small in scale to learn from the approach and iterate as it becomes more embedded. The focus of this financial year (2024-2025) will be to engage with stakeholders throughout this consultation process, to develop and establish the fundamental mechanics of the pathway. This includes the use of key enablers, levers and incentives, to provide clarity about health system priorities. This pathway will build a foundation for more value-driven decision making across medtech procurement and adoption within the NHS.
1.10 While the scope of the pathway applies to products assessed by NICE for use by the NHS in England, elements that support it have benefitted from key input of the devolved administrations, such as the Innovative devices access pathway. NHS England, NICE and the DHSC will continue to closely engage with devolved administrations going forward.
1.11 Section 2 of this document sets out the guiding principles underpinning the development of the pathway. Section 3 describes the key elements of the integrated, rules-based medtech pathway in more detail. Section 4 sets out our next steps. The engagement questions have been consolidated for ease in the appendix.
1.12 Responses to this consultation will be extremely helpful to develop our proposals for the pathway. A report summarising the feedback, along with NHS England and NICE’s response will be published after the engagement period.
2. Guiding principles
2.1 A set of principles have been developed to help ensure that the pathway as described can deliver the strategic aims of improving outcomes for patients, providing greater certainty for medtech innovators and suppliers, and driving better value for money for taxpayers and the NHS.
2.2 These principles articulate what the pathway is seeking to achieve, as well as its scope and constraints. They are designed to guide decisions and prioritisation when needed.
Principle 1 – the pathway should be supported by evidence-based advice and guidance from the National Institute for Health and Care Excellence (NICE), focused on technologies with the greatest impact on patient outcomes and the most compelling cases for clinical and cost-effectiveness
NICE should maintain its independent role of providing guidance and advice on the use of medtech throughout the NHS through a streamlined Healthtech Assessment Programme. Through evaluation and guidance, NICE will maximise the overall value of medtech to the population and the NHS, and the topic selection process will ensure that technologies are those that stand to have the greatest positive impact. Building on the NHS’ experience of commissioning medtech, including in relation to pathway transformation and implementation, NICE’s methods for medtech will evolve to not only assess clinical-effectiveness and cost-savings, but also incremental and comparative cost-effectiveness over time.
Principle 2 – the pathway requires a lifecycle approach to support new, early-stage technologies as well as driving greater value from existing technologies in widespread use
NICE’s Healthtech Guidance Programme spans the entire product lifecycle for medical technologies, and this will support clinical and cost-effectiveness assessments at all stages, building on the validity of NICE’s established health technology evaluation methods.
Principle 3 – the pathway should lead to automatic identification of funding to support routine commissioning and adoption for clinically and cost-effective and affordable technologies
New technologies in scope must support the sustainability of NHS finances and be considered alongside the opportunity cost of displacing other investments in NHS treatments and services. To ensure affordability for the NHS, the number of topics entering the pathway for assessment is likely to increase over time, but for those that are determined to be both clinically and cost effective and affordable there will be a commitment to automatic identification of funding to support routine commissioning of these technologies in the NHS – providing clarity on access for eligible patients as well as a clear incentive for industry to work in partnership with the health service and NICE. These technologies will be subject to a budget impact test, factoring in service and implementation costs and informing prioritisation of topics for assessment, commercial negotiations, and implementation timeframes. This will also reduce the need for repeated negotiations with multiple NHS organisations.
Principle 4 – the pathway should support the transformation of clinical pathways and services
The introduction of new medical technologies can necessitate far-reaching changes in clinical practice and services, such as reducing the need for outpatient appointments or radically reshaping diagnostic approaches. Introducing these technologies has the possibility to drive profound and innovative service redesign with real benefits for patients and the system. NICE will, therefore, take into account the potentially significant pathway transformation and implementation costs of innovations when considering the case for NHS commissioning. In addition, some innovative technologies recommended through the pathway will need sufficient time to deliver and will require phased pathway change and adoption.
Principle 5 – the pathway should drive up the quality and use of evidence, helping tackle ethnic and unfair biases in medtech
The pathway should increase both the supply of and demand for evidence in health innovations, supporting decision-making particularly where clinical evidence is currently limited. Lessons learned and evidence generated from wide-scale adoption of medtech will help supplement early analysis, pilots, and evidence gathered by manufacturers.
This will also support a system-wide drive to tackle unfair biases in medical devices, following on from the recent Equity in medical devices independent review and the priorities of NHS England’s Core20PLUS5 approach to reduce healthcare disparities.
- Question 1 – are there any other important principles that should guide the development of an integrated, rules-based medtech pathway?
- Question 2 – what positive or adverse impacts could the integrated, rules-based medtech pathway have on protected characteristic groups and people at particular risk of health disparities? How do you think those impacts should be addressed?
3. Developing an integrated, rules-based pathway
3.1 This section describes each step of the proposed pathway. The pathway builds on current progress to support innovation across NHS England, the National Institute for Health and Care Excellence (NICE), the Medicines and Healthcare products Regulatory Agency (MHRA), and the Department of Health and Social Care (DHSC).
3.2 There are 5 key phases to the proposed integrated, rules-based medical technology (medtech) pathway, set out in figure 1 below, that will apply to selected new technologies entering the NHS, as well as established technologies that continue to develop throughout their lifecycle:
- Pre-authorisation: this phase covers activities before a product is approved for sale in the market. It includes manufacturer idea creation and proposition development, as well as horizon scanning and demand signalling.
- Authorisation: this is about everything related to obtaining the necessary authorisation to take a product to market. It includes the process leading to registration with MHRA, following the appropriate assessment and certification processes.
- Evaluation and guidance: this is about determining whether a technology is a clinically and cost effective use of NHS resources, ultimately aiming for NICE evaluation and recommendation for use in the NHS.
- Commercial and commissioning: this covers how products are commissioned after being recommended by NICE. It includes funding and commercial negotiation at both national and integrated care board levels.
- Scaled adoption: this is about increasing widespread adoption and patient access to medtech. It includes workforce considerations, pathway changes and realising the benefits of medtech.
Figure 1: Integrated, rules-based medtech pathway
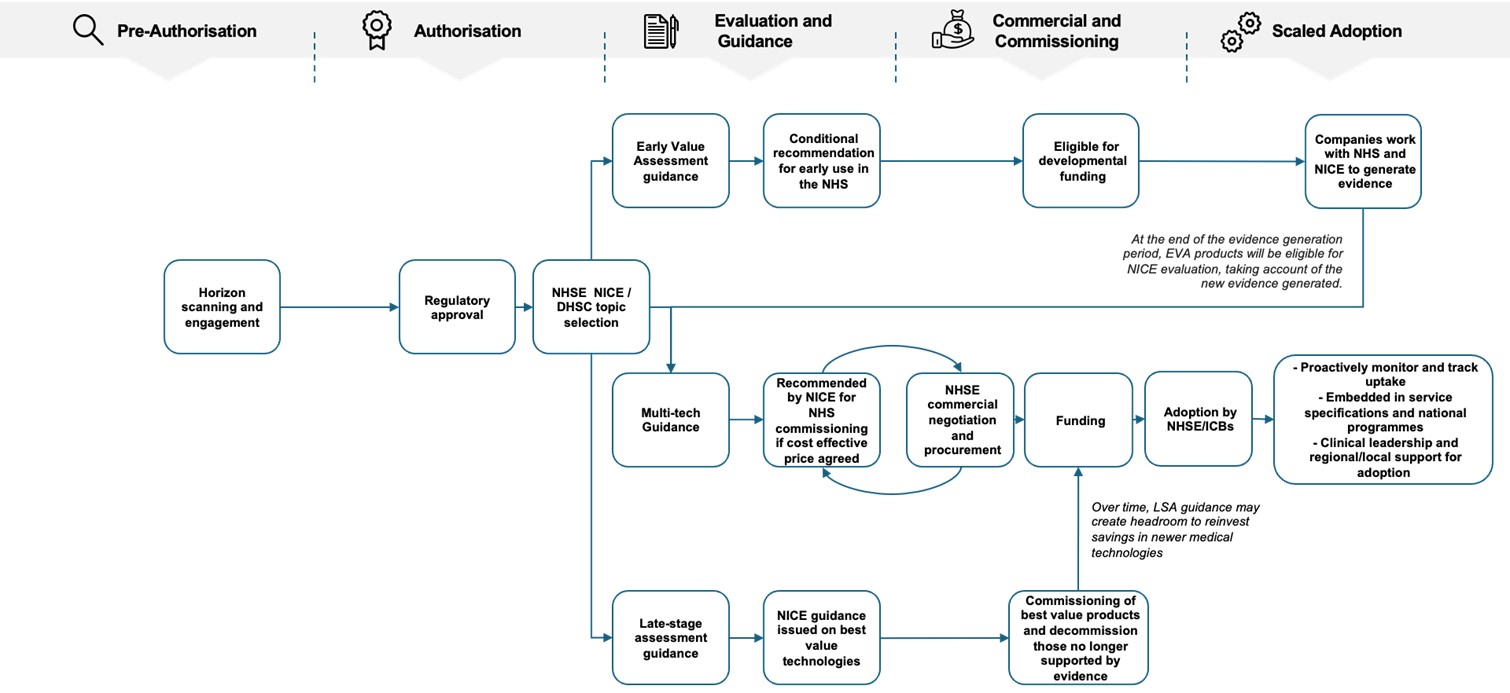
Figure 1 is a flowchart which summarises the proposed integrated, rules-based medical technology (medtech) pathway sequence. The sequence follows a pathway of pre-authorisation (horizon scanning and engagement), authorisation (regulatory approval), evaluation and guidance (including the National Institute for Health and Care Excellence’s guidance programmes), commercial and commissioning (including NHS England commercial negotiation and funding) and ends with scaled adoption (including clinical leadership and regional/local support for adoption).
Pre-authorisation
3.3 Effective horizon scanning and early engagement is essential. It allows the NHS to understand the most promising technologies in development and their likely impact on patients, existing services, and budgets. It also provides an indication of the structure of the market and maturity of new and emerging innovation, so that the NHS is ready to respond as markets evolve.
3.4 For medicines, there is a clear pipeline through which data can be accessed at defined points in the development cycle. There are also policies in place that encourage data sharing as a prerequisite to access the NHS. Building on this experience, NHS England and NICE want to enhance the approach to early engagement, demand signalling and horizon-scanning for medtech.
3.5 Work is happening in parallel across partner agencies, including MHRA, NICE, the National Institute for Health and Care Research Innovation Observatory, NHS Supply Chain, and the health innovation networks. Coordination of these various initiatives is critical to ensure that they combine to make the health system’s priorities for research and innovation clearer to medtech innovators. Given the volume of medtech solutions that are being created, it is vital that all elements of the medtech pathway are aligned to support the uptake of those innovations that will drive the greatest clinical and economic benefits.
Data for horizon scanning
3.6 To improve horizon scanning for medtech, there needs to be a more coordinated approach to data collection. This includes early proactive and structured engagement with industry, to allow the NHS to better understand the medtech pipeline. Building on how the NHS Innovation Service (NHS IS) has developed the Innovation record, there is ongoing work across organisations to agree the baseline-level of information needed for horizon scanning. The format of this information needs to be fit for purpose for both industry, and all end-users.
3.7 To enable this, NHS England will work with partner agencies to map disparate databases from across the health system; and to define and establish the necessary flow of information to enhance horizon scanning for medtech.
- Question 3 – do you agree that the timely and accurate provision of information by industry should be a pre-requisite for NICE evaluation?
- Question 4 – how could all partners work with industry to ensure data coming from emerging innovations is robust and supports high quality horizon scanning?
Early engagement
3.8 Early engagement will be essential to the pathway, providing an opportunity to share advice as well as signposting technologies through the pathway where they are most likely to benefit from a NICE assessment.
3.9 Efforts are already underway to improve horizon scanning, demand signalling and early engagement through developing the NHS IS as the ‘centralised front door’ for healthcare innovations that have potential use in the NHS. The NHS IS acts as an online platform that provides innovators with free expert information and coordinated, tailored support from relevant organisations. Innovators can communicate with and get support from MHRA, research bodies, Health technology assessment bodies, NHS Supply Chain and NHS England. This enables innovators to navigate the health and care system and deliver innovations faster. This is alongside work to improve market intelligence with better connected data systems.
3.10 Industry, sector, and wider engagement is essential for how the NHS defines and measures the value of medtech. For early product development, NICE assessment, commissioning decisions, and local benefits realisation there needs to be a shared view of value. NHS England’s commercial function will communicate with industry on how the NHS perceives the ‘value’ of medtech, considering feasibility and implementation alongside clinical outcomes. Over time this will help shape the medtech market more upstream, especially in emerging segments in which competition and pricing is less mature.
3.11 In addition, NHS England aims to develop ‘rough order of magnitude’ analysis for new products. Pre-authorisation this will focus on the likely scale of the clinical need, while post-authorisation and at the evaluation stage further analysis will support understanding of the potential affordability of innovations to the NHS within various subcategories.
- Question 5 – should the NHS Innovation Service provide any additional functionality to act as the ‘centralised front door’ for all innovative technologies in the NHS?
- Question 6 – how can stakeholders inform a shared understanding of the value of medtech to the NHS earlier in a product’s development cycle?
Demand signalling
3.12 NHS England, NICE and DHSC need to provide a coordinated signal to medtech innovators on the health system’s priorities for research and innovation. There must be a clear link then to the topic selection process and the work underway to improve demand signalling. Given the volume of new medtech, it is vital this pathway stimulates the uptake of those innovations that will drive the greatest clinical and economic benefits.
3.13 NHS England works with a wide range of stakeholders across the system, including the Accelerated Access Collaborative (AAC), people with personal experience, charities, academics, and industry to map out care pathways and review existing evidence to identify where further research and innovation is needed and improve systems alignment around these needs. These findings are shared on the AAC website and with AAC partner agencies and will soon be shared on the NHS IS website to evidence where targeted innovation might have the most significant impact.
3.14 To make clinical priorities for research and innovation clear and target horizon scanning towards the most impactful areas, NHS England will work with DHSC and NICE to triangulate current demand signals. Then, working with AAC partners, clinical leaders and industry, NHS England will develop an engagement strategy to quickly and clearly share the health system’s priorities.
- Question 7 – how can all partners better signal demand to industry, academia, innovators, and investors? What information channels should NHS England and the National Institute for Health and Care Excellence use?
Authorisation
3.15 Regulation is critical to the pathway, ensuring that patient safety is prioritised while enabling access to the most transformative technologies and supporting the UK’s innovation environment to flourish.
3.16 In January 2024, the MHRA launched its new roadmap (see figure 2). This sets out a clear path for developing new regulations for medical devices in the UK through a series of new statutory instruments. Priority measures to protect patient safety (through enhanced post-market surveillance) will be put in place in 2024, with other core elements of the framework expected to be in place in 2025.
Figure 2: Medicines and Healthcare products Regulatory Agency roadmap
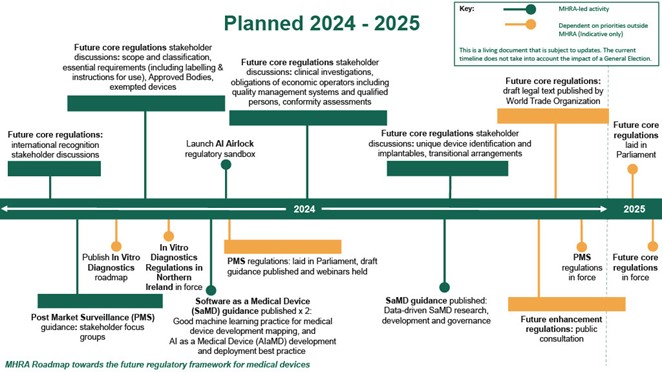
Figure 2 is a timeline of planned timescales for delivery of the future core regulations for the Medicines and Healthcare products Regulatory Agency (MHRA). The timeline cover the 2024 to 2025 time period, with activity plotted in chronological order.
- The following MHRA-led activity is included: future core regulations; future core regulations stakeholder discussions; post market surveillance (PMS) guidance; launch artificial intelligence airlock regulatory sandbox; software as medical device guidance published x 2; future core regulations stakeholder discussions; future core regulations stakeholder discussions; software as medical device guidance published.
- The following activity is included that is highlighted as being dependent on priorities outside MHRA: publish in vitro diagnostics roadmap (IVDR); IVDR-Northern Ireland regulations in force; PMS regulations: laid in Parliament, draft guidance published and webinars held; future core regulations: draft legal text published by World Trade Organization; future enhancement regulations: public consultation; PMS regulations in force; future core regulations laid in Parliament; future core regulations in force.
3.17 In line with the aims of the pathway, these new regulations will support the launch of the most transformative technologies and enable patients to access world-leading innovations both swiftly and safely.
3.18 The MHRA is also working alongside key partners in DHSC, Health Technology Wales, NHS England, NICE, the Office for Life Sciences, and the Scottish Health Technologies Group to deliver the Innovative Devices Access Pathway (IDAP) pilot. IDAP aims to accelerate patient access to transformative devices that support an unmet need, through a streamlined regulatory and access pathway for developers. Having been announced in February 2024, 8 new medical technologies that do not yet have regulatory approval have been selected for the pilot. The insights and learning gathered throughout the process will feed into how IDAP develops over the coming years. It will also provide insights for further development of this overarching medtech pathway.
Evaluation and guidance
Topic selection and prioritisation
3.19 NICE has now consulted on its integrated topic prioritisation and strategic principles, streamlining the current NICE topic selection routes and making pathways more predictable and more reflective of NHS priorities.
3.20 The proposal is to introduce a new overarching approach to topic selection and prioritisation that better integrates all NICE’s outputs. This will see the creation of a centralised prioritisation board that will make topic selection decisions for all NICE programmes.
3.21 Topic selection will be integrated with the horizon scanning and demand signalling activity described above. As well as engaging with the NHS IS, NICE’s topic selection consultation describes a range of sources to identify topics that meet the priorities of the health and care system.
3.22 The NICE prioritisation board will share details on all topics under consideration with relevant clinical and policy teams across DHSC and NHS England, and carefully consider feedback received before making its decisions. In addition, DHSC and NHS England can specifically request topics for the prioritisation board to consider.
3.23 To decide on priority topics NICE have developed a set of eligibility as well as decision making criteria, covering:
- budget impact
- system impact
- population impact
- evidence quality
- health inequalities
- environmental sustainability
3.24 Using these criteria the NICE prioritisation board will agree the relative priority for NICE to develop guidance in that topic area as well as the appropriate guidance product as discussed below.
3.25 For the pathway, these topics will be selected by NICE, NHS England and DHSC. This is akin to the approach used for routing topics for technology appraisal guidance and highly specialised technology guidance, where technologies must meet specific eligibility criteria to be selected for assessment.
3.26 NHS England and NICE anticipate a limited number of technologies will be assessed through multi-tech guidance (MTG) for routine commissioning, funding and adoption per year in the pathway’s early years, as many technologies, such as early value assessment (EVA) products, are not likely to have sufficient evidence for a robust health technology assessment. This will mean that this route will only apply to medtech that has strong evidence indicative of significant benefit to the health service, among the wider criteria referenced in this document. Initially, the expectation is that around 5 products or groups of products will be assessed by NICE through MTG for the new pathway.
3.27 Topic selection and prioritisation will also ensure that technologies that come through the pathway are those that offer the greatest clinical and economic benefits. For medicines, increased costs are being supported by the Voluntary Scheme for Branded Medicines Pricing, Access and Growth, but no equivalent exists for medtech. To ensure the affordability of this route, NICE and NHS England will estimate the potential budget impact of a technology, including service and implementation costs, at the topic selection and prioritisation stage. This will include the likely impact on health and care systems budgets of implementing the potential topic guidance.
3.28 This approach will improve shared planning, send a clear signal to industry about NHS priorities and ensure that limited resources are focused on the most promising medtech.
- Question 8 – What additional factors should NHS England, the National Institute for Health and Care Excellence and the Department of Health and Social Care consider when selecting technologies and categories of technologies for the pathway?
National Institute for Health and Care Excellence healthtech assessment
3.29 NICE has introduced several different routes to assess the clinical benefit of a technology after topic selection which span the lifecycle of technology development: EVA, multi-technology guidance (MTG) and late-stage assessment (LSA). There are clear, rules-based eligibility criteria for each.
3.30 These different routes ensure NICE can assess medtech across the entire product lifecycle to ensure best use of taxpayers’ money. NICE is also ensuring that all its advisory bodies can undertake the full spectrum of methods for clinical and cost effectiveness analysis and that they can be tailored to each specific problem.
Figure 3: National Institute for Health and Care Excellence’s guidance programmes span the product lifecycle for medical technologies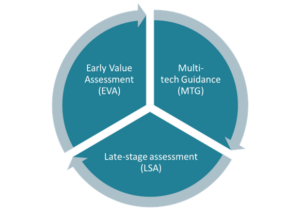
Figure 3 is a pie chart which shows the National Institute for Health and Care Excellence’s guidance programmes. It has 3 segments: early value assessment (EVA); multi-tech guidance (MTG); late-stage assessment (LSA).
3.31 NICE EVA guidance considers technologies that address national unmet need, rapidly assessing products early in the lifecycle that need further evidence to support wider adoption.
3.32 EVA is similar in principle to a managed access recommendation for medicines. It provides a conditional recommendation for use (acknowledging there is uncertainty due to limited evidence) while further evidence is being generated. This enables early access to promising new technologies for patients. Subject to data collection, the technology should then receive a full NICE MTG assessment and NICE guidance, supporting a lifecycle approach.
3.33 For industry, EVA guidance will:
- offer a faster and more streamlined route to a NICE assessment for conditional recommendation for use in the NHS
- clearly identify the additional evidence needed for a full NICE appraisal and recommendation on future routine use in the NHS
- facilitate greater market adoption ahead of full NICE guidance
- act as a ‘bridge’ to a full NICE MTG appraisal, with NICE’s conditional recommendation for use in the NHS enabling the technology developer to gather the necessary evidence.
3.34 For NHS clinicians and commissioners, EVA guidance will:
- identify technologies that address patient need and clinical demand, and prepare for the most promising technologies that have the potential for high impact and likelihood of adoption
- provide an independent assessment of evidence of clinical and cost effectiveness to inform the adoption of the technology
- provide evidence to indicate whether the benefits of a technology are expected to be realised in the NHS. In turn, further real-world evidence can be produced from multiple sites, offering a greater level of assurance of the evidence base
- help the NHS to use financial resources more effectively
- facilitate earlier access to promising technologies, while minimising any clinical and system risk.
3.35 Products that receive positive EVA guidance will be conditionally recommended for use in the NHS, where clinical and system risk can be managed. Receiving a conditional recommendation should increase adoption of the product within the NHS, while further evidence of patient outcomes and system benefits are generated to support the potential future routine use of the technology. The length of a conditional recommendation will be determined on a product-by-product basis but is expected to last for 2 to 3 years. At the end of the evidence generation period, the technology will be eligible for reappraisal by NICE, taking account of the new evidence generated.
3.36 Products that are conditionally recommended for use in the NHS through EVA guidance will have an accompanying evidence generation plan. This outlines the evidence gaps and what real-world evidence needs to be generated for NICE to reappraise the product, with a focus on potential patient and system benefits in routine practice.
3.37 The NICE MTG programme assesses innovative products that have not yet been widely adopted but have substantial evidence to produce guidance in favour or against use in the NHS. This is based on an assessment of clinical and cost effectiveness.
3.38 The programme evaluates new, innovative medical devices and diagnostics. It looks at medtech that deliver treatment (like those implanted during surgery), give greater independence to patients, and detect or monitor medical conditions.
3.39 NICE’s advisory committees make guidance decisions with the aim of facilitating the uptake of cost-effective and cost-saving technologies in the NHS.
3.40 NICE committees have used different medtech evaluation methods to the approach in this pathway. The current criteria for the Medtech funding mandate policy (MTFM) requires technology to demonstrate cost-saving potential within three years to be supported by MTFM for use within the NHS. This requirement can be challenging for technologies that offer significant benefits to patients but cannot prove they are cost saving quickly, and it may be too high a hurdle for technologies that require significant investment before their full value for patients and the system can be realised.
3.41 In future, NICE will consider the cost-effectiveness and value offered by both cost-incurring as well as cost-saving medtech, using the principles described in the previous section. NICE will use their standard cost-effectiveness threshold. In general interventions with an incremental cost-effectiveness ratio of less than £20,000 per quality-adjusted life year gained are considered to be cost effective by NICE. The outcome of MTG will be a recommendation from NICE on the cost-effectiveness of a category of technologies. NICE may also issue a negative MTG recommendation which means that the case for adoption is not supported, or a ‘research only’ recommendation which means that further evidence is needed.
3.42 Based on this, for those that technologies that have received a positive MTG recommendation for routine use in the NHS, NHS England will engage in a commercial negotiation and procurement exercise. Technologies positively recommended should have a budget impact of no more than £10 million per year (considering commercial negotiations and cost savings from introducing the technology) to be eligible for automatic identification of funding to support routine commissioning. For MTG-recommended products with a greater budget impact, the commercial negotiation and procurement exercise may consider how best to support more gradual adoption over time, balanced against affordability constraints.
3.43 NICE LSA guidance will apply to categories (or classes) of products that are already in widespread or established use within the system.
3.44 Many technologies in use in the NHS have not had a formal assessment of cost effectiveness. While it would be impossible to assess all products, LSA guidance will assess those that are used in sufficient volume to ensure that they are cost effective, and that best value can be secured across the category.
3.45 LSA guidance will assess technologies that are already in widespread or established use in the NHS to inform commissioning and procurement decisions. Technologies in use often undergo continuous or incremental innovation and adaptation. LSA guidance will assess whether the products within a category of technologies, currently chosen by DHSC represent value for money, and whether price variations are justified by the incremental differences and advancements.
3.46 LSA guidance will also evaluate differences between products to determine whether price variations from the benchmark and between products are justified. This will enable procurement services to make well-informed decisions.
- Question 9 – how can products that receive a positive early value assessment recommendation best be supported to develop evidence?
- Question 10 – to what extent do you think there is an opportunity to streamline existing innovation funding streams to provide a more systematic approach to supporting conditional reimbursement for early value assessment recommended medtech?
Commercial
3.47 Commercial activities will be embedded throughout the pathway, incorporating 3 distinct areas of commercial activity:
- planning and defining NHS need
- assessing the most viable sourcing model
- agreeing the means for implementation and management, in particular how to consistently scale medtech adoption.
3.48 The objectives of commercial activities across the pathway will be to maximise the value of these medtech products to the NHS (based on NHS priorities) and provide greater certainty to industry around how value will be understood and measured, so that they can provide products which align with this. This should be underpinned by a clear, agreed view of what the NHS defines as benefit from medtech, which can be applied to activities from pre-authorisation through to scaled adoption.
3.49 Alongside the NICE evaluation of MTG products, commercial teams will explore different payment and sourcing models to secure a cost-effective and affordable price. medtech suppliers and associated trade bodies will in turn have greater clarity on the cost-effectiveness of their value proposition from the perspective of NICE and the NHS. To support this, NHS England will combine best practice from the Cabinet Office Government Commercial Function with market specific commercial and procurement processes.
3.50 NHS England’s commercial approach will incorporate the following commercial activities:
- Early assessment of affordability, in parallel to horizon scanning activities and building an understanding of emerging medtech markets through proactive engagement with suppliers to actively shape the product pipeline.
- Early budget analysis, considered alongside patient and system impact, as part of topic selection processes and early stages of evaluation delivered jointly by NICE and NHS England to embed commercial sustainability as a key criterion for prioritisation and assessment of medtech.
- Early identification of payment models and funding mechanisms aligned to different elements of the pathway (EVA; MTG; LSA).
- Alongside NICE assessment processes, applying commercial negotiation and sourcing approaches to secure the most affordable price for the NHS while being sustainable for industry and maintaining competition. This will consider a number of factors in parallel to NICE assessment including; scale of need and volume of product required; purchasing commitments over time; ease of implementation of medtech across pathways; projected costs and benefits associated with widespread adoption; and proliferation of similar products on the market.
- Providing industry with clear sourcing and procurement mechanisms for recommended products which show clinical and financial value will be a priority. To minimise complexity and maximise competition through proven approaches, existing procurement infrastructure will be leveraged to best effect, incorporating clear roles for established sourcing routes approved by the NHS England Commercial Function. Utilising current mechanisms will also help to lay the foundations to maximise value for money from all medtech, beyond the first year of the pathway. Selection of an appropriate sourcing mechanism will be dependent upon a number of factors, including the clinical and cost impact of medtech products, and how they will be implemented and accessed by clinicians and patients.
- Guidance and support for local systems to realise and capture the benefits and longer-term value of medtech. This will include performance and quality management of products and contracts, with a mechanism to share insights from real-world implementation to inform horizon scanning and further shape the market upstream. Utilising established procurement routes will help in providing a central data source related to performance and quality through the adoption of medtech.
- Question 11 – do you envisage the proposed commercial activities will help the NHS to maximise value for money from new medtech?
- Question 12 – please provide comments on what, if any, other commercial mechanisms/activity NHS England and NICE should consider to maximise value for money from medtech through the pathway.
- Question 13 – what further work could help to inform an understanding of the value of medtech to support sustainable commissioning, funding, and adoption through the pathway?
Commissioning
3.51 The medtech pathway aims to provide a consistent set of ‘rules’ that will be applied to suitable funding streams whether they are held nationally or by integrated care boards (ICBs), based on a range of potential funding sources, to deliver access and adoption.
3.52 For EVA guidance, established programmes of funding may be linked to a recommendation in EVA guidance to provide support while evidence is generated. NICE will work with the technology developer to identify system partners who can support the delivery of evidence generation. NICE can also broker relationships, as needed, with potential data controllers and research funders. We are seeking views on streamlining existing innovation funding streams to provide a more systematic approach to supporting conditional reimbursement.
3.53 For MTG recommendations, the introduction of the pathway will better support the effective use of nationally held, ringfenced funds to scale the adoption and rollout of well-evidence, beneficial medtech, informed by technologies receiving a positive NICE MTG recommendation. When a fund is for a specific purpose or category of medtech, the technology will also need to show that it meets the established eligibility criteria of the fund as well, for example, by reducing economic inactivity or reducing waiting lists.
3.54 Where medtech falls within an ICB-commissioned pathway and has been recommended by NICE, the pathway will support trusts and ICBs in making decisions about what to commission and fund by providing guidance on technologies that are clinically and cost effective. NHS England can support this through their business as usual arrangements, including planning guidance and adoption support.
Scaled adoption
3.55 NHS England and ICBs use NICE’s evidence based advice and guidance to commission medicines and technologies for the benefit of their patients and staff. The pathway will provide a consistent and predictable route for commissioners to adopt new technologies to improve care and clinical pathways based on a NICE recommendation.
3.56 Over time, the pathway will support a shift to a more rules-based approach to adoption – with EVA guidance and an MTG providing robust, evidence-based guidance to commissioners on new technologies. Equally, if technologies are already in widespread use or established within the system, LSA guidance will help drive efficiencies and secure value for money in procurement decisions – or in some cases, may indicate when a technology could replace an old procedure or when decommissioning treatments is the most appropriate route. NICE recently launched a consultation on their interim LSA methods and processes. NICE will publish the outcome of this consultation in quarter 2 2024.
3.57 For specialised services, new technologies will need to be embedded into service specifications for specialised services. National clinical and commissioning teams will need to update clinical guidance to ensure it reflects this, detailing the intervention, evidence summary, and approach to implementation.
3.58 For ICB-commissioned services, ICBs will be expected to update their own service specifications and clinical commissioning policies in line with NICE guidance.
3.59 Commissioners also have a responsibility to ensure that there is consistent uptake in new technologies and to reduce variation across England. With the introduction of the pathway, there is an opportunity to support a data-driven, evidence-based approach to monitor and track uptake of these new technologies proactively, including through the ongoing development of data infrastructure. Where appropriate, NHS Supply Chain and other procurement partners will play a leading role in developing and strengthening effective catalogue management to monitor adoption.
3.60 Clinical leadership and staff training will also be key to widespread adoption of new innovative medtech. NHS England and ICBs will need to utilise their existing clinical networks – which bring together clinical experts, patients, and other partners to help drive improvements against national clinical priorities – to encourage and support adoption and uptake.
3.61 Decommissioning will be a key activity linked to NICE’s LSA guidance to maximise the value of medtech for the NHS. This will be informed by benefits and value realisation from medtech implementation and the extent to which it is embedded over time.
3.62 Further measures to encourage adoption will be taken in line with the recommendations of the Innovation ecosystem review.
4. Next steps
4.1 The proposals set out in this consultation document have been developed by NHS England and the National Institute for Health and Care Excellence (NICE), in partnership with the Department of Health and Social Care (DHSC), the Medicines and Healthcare products Regulatory Agency (MHRA) and other partners. The consultation period will be key in testing and further developing the proposals, so that they can be iterated, adapted, and further refined ahead of the new pathway being piloted later in the year.
4.2 The consultation period will remain open for responses until midnight on Thursday 15 August 2024. Engagement questions are set out alongside each chapter and can also be found in the appendix. Full instructions for how to respond can be found in the how do I make my views known section of this document.
4.3 NHS England and NICE will jointly run a series of webinars that will include a presentation, highlight key engagement points, and provide an opportunity for questions and discussion. These will be a mix of general sessions and more targeted sessions for specific groups, including industry, NHS trusts and integrated care boards. Details of the webinars will be shared on the NHS England events page.
4.4 Following the engagement period, all feedback will be considered and reflected upon before a response and final plans for implementation from NHS England and NICE is published. Thank you for taking the time to read and respond to this document.
4.5 For any enquiries about the proposals, please contact: medtechpathwayqueries@nhs.net
Appendix: consultation questions
Section 2: guiding principles
- Question 1 – are there any other important principles that should guide the development of an integrated, rules-based medtech pathway?
- Question 2 – what positive or adverse impacts could the integrated, rules-based medtech pathway have on protected characteristic groups and people at particular risk of health disparities? How do you think those impacts should be addressed?
Section 3: pre-authorisation
- Question 3 – Do you agree that the timely and accurate provision of information by industry should be a pre-requisite for National Institute for Health and Care Excellence evaluation?
- Question 4 – How could all partners work with industry to ensure data coming from emerging innovations is robust and supports high quality horizon scanning?
- Question 5 – Should the NHS Innovation Service provide any additional functionality to act as the ‘centralised front door’ for all innovative technologies in the NHS?
- Question 6 – How can stakeholders inform a shared understanding of the value of medtech to the NHS earlier in a product’s development cycle?
- Question 7 – How can all partners better signal demand to industry, academia, innovators, and investors? What information channels should NHS England and the National Institute for Health and Care Excellence use?
Section 3: evaluation and guidance
- Question 8 – what additional factors should NHS England, the National Institute for Health and Care Excellence and the Department of Health and Social Care consider when selecting technologies and categories of technologies for the pathway?
- Question 9 – how can products that receive a positive early value assessment recommendation best be supported to develop evidence?
- Question 10 – to what extent do you think there is an opportunity to streamline existing innovation funding streams to provide a more systematic approach to supporting conditional reimbursement for early value assessment recommended medtech?
Section 3: commercial
- Question 11 – Do you envisage the proposed commercial activities will help the NHS to maximise value for money from new medtech?
- Question 12 – Please provide comments on what, if any, other commercial mechanisms/activity NHS England and the National Institute for Health and Care Excellence should consider to maximise value for money from medtech through the pathway.
- Question 13 – what further work could help to inform an understanding of the value of medtech to support sustainable commissioning, funding, and adoption through the pathway?
Publication reference: PRN01328

