1. Introduction
The national Community Diagnostic Centre (CDC) Programme is now in its third year and has approved 170 CDC sites across England. As at August 2024, 165 sites are operational in a variety of settings including shopping centres, university campuses and football stadiums. Of these, 135 are operating from their permanent CDC building and the rest from temporary capacity while the CDC build is completed.
They offer patients a wide range of diagnostic tests closer to home and greater choice on where and how they are undertaken, reducing the need for hospital visits and potentially expediting the start of treatment. So far these centres have delivered over 9 million tests, checks and scans.
The focus now is the full opening and development of these sites so that they, along with new investment in acute imaging and endoscopy services, can deliver up to 9 million tests each year by the end of 2024/25; and to meet the 6 primary aims of the CDC Programme and related cross-cutting themes, as illustrated in Figure 1 below.
This guidance sets out next steps and best practice for systems on developing CDCs, including sections on commissioning, governance, engagement, regulatory and accreditation requirements, digital connectivity, workforce and pathway development. It updates and replaces the guidance published on the CDC FutureNHS page in June 2022.
Figure 1: CDC Programme aims and cross-cutting themes
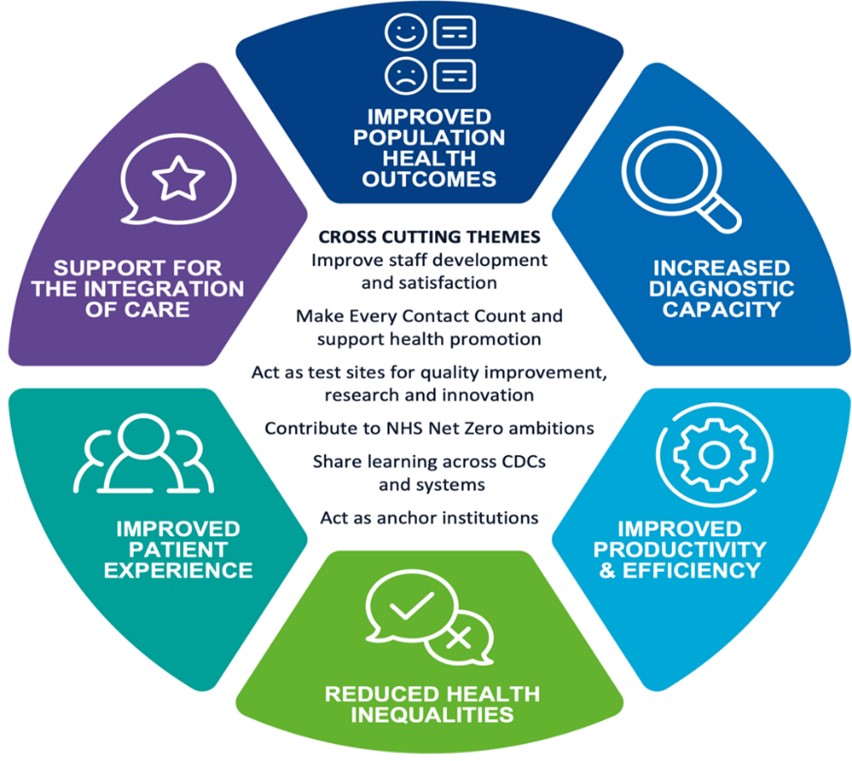
The diagram is divided into six segments, each representing a different aim or theme.
The segments are labelled as follows:
- improved population health outcomes
- increased diagnostic capacity
- improved productivity and efficiency
- reduction in health inequalities
- improved patient experience
- support for the integration of care
In the centre, there is a section labelled “Cross-Cutting Themes,” which includes the following points related to the themes:
- make every contact count and support health promotion
- act as test sites for quality improvement, research and innovation
- contribute to NHS net zero ambitions
- share learning across CDCS and systems
- act as anchor institutions
In further establishing and embedding CDCs, systems must also take account of the NHS 2024/25 priorities and operational planning guidance.
For any queries about this guidance or to provide feedback, please contact us: england.cdcprogramme@nhs.net.
2. What is a community diagnostic centre?
CDCs deliver additional, digitally connected, diagnostic capacity in England, providing all patients with a co-ordinated set of diagnostic tests in the community and in as few visits as possible, enabling an accurate and fast diagnosis on a range of clinical pathways.
CDCs provide a broad range of elective diagnostics away from acute facilities, reducing pressure on hospitals and giving patients quicker and more convenient access to tests. CDCs co-ordinate all the diagnostic tests a patient requires and, wherever possible, provide them under one roof in a single visit.
Location and operating hours
A CDC must be located away from other acute hospital services on a separate site. The exception to this is CDCs built in year 1 or 2 of the programme where co-location with, but through a separate entrance from, the acute hospital was permitted to support elective recovery at pace.
CDCs should provide services for at least 12 hours a day, 7 days a week. Systems should ensure they have plans in place to support their CDCs to achieve this requirement.
CDC design archetypes
CDCs must align with 1 of 3 broad design archetypes:
- standard model: Provides at least the minimum diagnostic tests (listed in Table 1) on a permanent basis and on the same site, as well as other components of the diagnostic pathway such as outpatient consultations. It may also provide other suitable diagnostic tests deemed to be a priority locally
- large model: As well as meeting all the requirements of a standard CDC, it offers endoscopy and/or any other services/tests required locally (for example, ophthalmology, audiology, health checks and screening services) at scale. Scalability is an important feature of a large CDC; for example, centres may have multiple scanners to improve efficiency/effectiveness
- hub and spoke model: The central ‘hub’ is a standard or large model CDC, and each spoke is an approved facility in a community healthcare setting, a commercial setting or another non-acute healthcare provider setting. A spoke must have 1 core diagnostic imaging modality (CT, MRI, X-ray or ultrasound) plus provide at least 2 other tests from the minimum CDC test list (Table 1). It must have a defined location and be able to report its activity
These models are designed to ensure CDCs conform to a set of common standards, delivering long-term value for money while giving systems the flexibility to tailor local facilities to meet local needs.
CDCs must add local diagnostic capacity and support the delivery of wider strategic developments, including relevant local and national programmes (for example, cancer, elective recovery, outpatient transformation, new hospitals and elective surgical centre programmes). To facilitate this and support pathway development, CDCs should include outpatient consultation clinic rooms to be used flexibly across specialties. Integrated care boards (ICBs) are reminded of the need to prioritise new diagnostic capacity for cancer services.
Minimum test requirements for a standard CDC
A CDC must provide the agreed minimum set of core diagnostic tests on a permanent basis.
Table 1: Minimum core tests for a standard CDC
|
Diagnostic modality |
Minimum requirements |
|
Imaging |
CT MRI Ultrasound Plain X-ray |
|
Pathology |
Phlebotomy NT-proBNP Point of care testing (POCT) including for some of the following: eGFR, pro-BNP test, anticoagulation change of dose, C-reactive protein, hCG pregnancy test, urinalysis |
|
Physiological science |
12-lead electrocardiography (ECG) Ambulatory electrocardiography (for example, Holter, patch monitor) Ambulatory blood pressure monitoring Trans-thoracic echocardiogram (TTE) Spot check pulse oximetry POCT capillary blood gas assessment Fractional exhaled nitric oxide (FeNO) Spirometry with bronchodilator response Full lung function tests (lung volumes, gas transfer and spirometry) Field tests – 6-minute walk test Sleep studies – multichannel home recordings CO monitoring |
|
Endoscopy (large CDCs only) |
Gastroscopy – trans-nasal endoscopy (TNE) and/or oral oesophago-gastro duodenoscopy (OGD) Colonoscopy Flexi sigmoidoscopy |
All CDC sites, including spoke sites, are expected to undertake both contrast and non-contrast examinations across all tests as approved by the Resuscitation Council.
Optional additional tests
Systems should not feel limited to providing the minimum set of tests. Table 2 identifies non-core tests additional to the national minimum requirement that are suitable for CDC delivery, and which systems may want to consider when planning which services to include in their local CDC models. This is a non-exhaustive list, and systems are encouraged to provide additional tests from CDCs where these are deemed appropriate and a priority locally, and can be supported by local funding. Screening services can also be considered, if agreed and funded locally with screening commissioners.
Table 2: Optional tests additional to the minimum tests
|
Diagnostic modality |
Test |
|
|
Imaging |
Mammography DEXA scan Dermoscopy Elastography (for example, fibroscan) CT colonography | |
|
Physiological science |
Urodynamics Ophthalmology services Audiology services Non-complex neurophysiology (for example, for carpal tunnel syndrome) | |
|
Pathology |
Simple biopsies that are outpatient in nature – for example, skin and breast – and appropriate to the pathways and services being offered While all CDCs should provide phlebotomy and POCT, other samples can be taken on CDC sites but should be processed through existing pathology routes |
|
|
Endoscopy |
Colon capsule endoscopy Sponge on a string Cystoscopy Flexible cystoscopy Hysteroscopy Colposcopy | |
Tests unsuitable for CDCs
National clinical advisors have identified some tests as clinically unsuitable for delivery in a CDC setting, and they may continue to add tests to this list:
- endoscopic retrograde cholangiopancreatography (ERCP) and associated procedures
- complex interventional procedures including biopsies of internal organs (the exception will be local anaesthetic transperineal prostate biopsy [LATP])
- trans-oesophageal and stress echo
- bronchoscopy and endobronchial ultrasound (EBUS)
- cardiopulmonary exercise tests (CPET)
- complex sleep studies that include electroencephalography (EEG) monitoring
- needle electromyography (EMG)
- treadmill tests
- bronchial challenge tests (methacholine, mannitol and histamine) cold air challenge / exercise induced asthma tests
- manometry
Services for children and young people
Systems are expected to offer services for children and young people locally within CDCs where it is safe and appropriate to do so; for example, but not limited to, supporting physiology pathways for asthma and audiology. Systems must ensure that all relevant governance and safeguarding arrangements for children are in place, and that all relevant policies, processes and procedures are complied with. Where it is not appropriate or safe for a CDC to offer services to children and young people, systems should develop alternative plans to increase diagnostic capacity for them.
The physical environment and safeguarding measures in CDCs should be equivalent to those in primary care. There is no requirement to segregate adults and children, but CDCs should give thought to the waiting environment from a child’s perspective, in particular the provision of toys and play materials. Further information is available in NICE guidance [NG204] Babies, children and young people’s experience of healthcare.
There is no increased safeguarding training requirement over and above the mandatory children and young people safeguarding training all NHS staff undertake. All CDCs should have a named safeguarding lead (this can be the same as the host trust lead).
Utilisation rates and minimum activity levels
All CDC sites are expected to be performing at, or above, the activity levels given in Appendix A (unless rooms are shared between services) to maintain throughput and ensure efficiency. Where a CDC site introduces a new test for which training on equipment is required, the minimum required activity level should be achieved within 12 weeks of it going live. Mobile services for CT and MRI are expected to perform at the same levels as static services.
The required minimum annual activity levels by CDC archetype are:
- large CDC: 60,000 tests
- standard CDC: 50,000 tests
- spoke CDC: 25,000 tests (except where a spoke site has modalities that cannot deliver high volumes; for example, endoscopy, CT and MRI, rather than phlebotomy, POCT, etc)
3. Implementing community diagnostic centres
Commissioning and service models
Systems are required to commission their CDCs using the NHS Standard Contract. This also applies to any sub-contracting arrangements. However, systems are free to select the most appropriate commissioning and contracting model to meet local needs, making sure that their choice has no inherent single point of failure or overt reliance on one provider within a system or region.
Systems must take the lead in deciding which CDC archetypes will most benefit their area and identifying suitable provider arrangements (including host providers). NHS England has developed a national CDC framework of pre-qualified providers of CDC services (covering imaging, endoscopy, physiological science and pathology). Commissioners may draw down services from the framework, whether end to end (all modalities) or just one.
The CDC Programme is working closely with the NHS England Costing and Pricing team to refine the finance model for allocation of revenue for CDC activity. This is based on a cost per test and payment by results methodology and, while it will be reviewed annually, we expect 2024/25 revenue payments for each CDC to adhere to the 2024/25 Revenue funding policy for CDCs.
In 2024/25 the CDC Costing and Pricing team will progress a review of all collected cost per test data to inform 2025/26 proposed system uplift payments and, potentially, also future ICB baseline allocation adjustments from 2025/26.
ICBs should refer to NHS community diagnostic centres: Finance and contracting arrangements for 2024/25.
Independent sector (IS) providers are part of the delivery model for CDCs, and NHS, IS and joint NHS/IS sites are defined as follows:
- NHS site: one where the site is owned or leased by the NHS and all services are run by the NHS
- IS site: one where the location is owned or leased by the IS provider and most of the services delivered are run by the same IS provider
- Joint IS/NHS site: one where the NHS owns or leases the site but more than one of the tests delivered are run by the IS on the site
Governance, performance and engagement
We recommend that governance arrangements for CDCs reflect those of the host organisation and corresponding ICB. CDCs must comply with all relevant host trust frameworks, policies and procedures.
- CDC sites where applicable must be Care Quality Commission (CQC) registered. They must aim to meet accredited standards for their modalities within 2 years of being fully operational. The regulatory and accreditation requirements for core and optional services within CDCs are detailed in Table 3.
ICBs will monitor governance for all aspects of CDC delivery. We request that a monthly diagnostic board is held at system level to review all matters pertaining to the governance and performance of CDCs. Issues should be escalated, and successes and performance communicated to a regional diagnostics board and through to the National Diagnostics Board, by exception.
Table 3: CDC regulatory and accreditation requirements
For core tests
Diagnostic modality | Regulatory and accreditation requirements |
|---|---|
|
Imaging |
QSI – Quality Standards for Imaging Ionising Radiation (Medical Exposure) Regulations (IR(ME)R |
|
Physiological science |
This service must meet the regulatory and accreditation requirements set by UKAS: IQIPS- Improving Quality in Physiological Services |
|
Pathology |
ISO 15189 – Medical laboratory accreditation (UKAS) – for the linked laboratory for phlebotomy ISO 22870 – Point of care testing (UKAS), applied in conjunction with ISO 15189 |
|
Endoscopy (larger CDCs only) |
Joint advisory group on gastrointestinal endoscopy (JAG) Any CDC endoscopy service undertaking bowel cancer screening should operate in accordance with the Bowel Cancer Screening (BCSP) Programme Quality Standards with those standards set and monitored by the Screening Quality Assurance Service (SQAS) |
For optional additional tests
|
Any CDC providing breast screening should operate in accordance with the National Breast Screening Programme Quality Assurance Standards with those standards set and monitored by Screening Quality Assurance Service (SQAS) |
|
Any CDC providing diabetic eye screening should operate in accordance with the Diabetic Eye Screening Programme Quality Assurance Standards with those standards set and monitored by SQAS |
|
Any CDC providing colposcopy as part of cervical screening should operate in accordance with the Cervical Screening Programme Quality Standards with those standards set and monitored by SQAS |
|
Any CDC providing gynaecology services should operate in accordance with the CQC Additional Service Gynaecology and Termination of Pregnancy Framework |
CDCs must align with the review and management of performance process set out at national level (see 2024/25 Revenue funding policy for CDCs). This requires their timely communication to NHS England of changes to funded plans, scope of services, underperformance, delays and reporting issues, to ensure there is clarity, assurance and communication to meet the needs of the programme.
Access to CDCs should be integrated across all trusts within an ICB, with a single patient treatment list (PTL) used to funnel the patients waiting longest to CDCs, to speed up diagnosis and reduce waiting lists.
Estates
All CDC capital has been allocated in 2024/25 so any further work to identify new sites will be limited. If an identification process is required for new sites, systems should refer to the 2022 guidance..
As aerosol transmission of respiratory infections during lung function testing can be a risk to staff and patients, mitigating this needs to be a consideration for the estate and spaces these tests are performed in. Negative pressure ventilation, HEPA filters and ultraviolet air scrubbers can all reduce the risk. Guidance is available from the Association of Respiratory Technology and Physiology (ARTP).
Digital infrastructure and connectivity
The success of CDCs depends on the ability to share appropriate information and therefore the digital connectivity between diagnostic IT systems and organisations across healthcare systems.
Systems are required to ensure that connected and interoperable digital services are in place within the CDC delivery model to share imagery, clinical information and data to support clinical pathways and reporting for DM01 and the CDC portal. Relevant local networks (including imaging, physiological sciences, pathology, endoscopy and cancer networks/alliances) should be engaged to support data sharing and consideration of how they can best use CDCs to drive system-wide digital interoperability.
Unified solutions should be deployed to allow centralised CDC booking and image sharing, as well as to enable co-ordination of tests to deliver on complete pathway needs and offer patients a more personalised experience. Technology such as robotic process automation should be considered for scheduling to help reduce did not attends (DNAs) and improve utilisation of capacity alongside operational performance improvements.
Systems are required to progress deployment of iRefer and/or other decision support tools to facilitate the expansion of GP direct access and optimise capacity by ensuring the right examination is requested and inappropriate repeat testing is avoided.
Innovation driven by AI capability should be actively explored to support CDC processes and pathways.
Workforce
Systems should have robust recruitment and retention programmes to enable the development of a skill mix that can deliver the range of CDC services safely and effectively. This includes the use of shared roles across cardiology and respiratory, ensuring administrative roles can schedule across all modalities, and the use of alternative roles; for example, reporting radiographers, reporting healthcare scientists, extended paramedic roles and patient navigators. Resources to support workforce recruitment and retention are available via the Diagnostic Transformation Workforce FutureNHS page.
We recommend that staff based in CDCs rotate between community and acute sites (including IS services); for example, through workforce sharing agreements, secondments, digital staff passporting or joint staff banks. This supports training, recruitment and retention, and the health and wellbeing of staff.
In developing their workforce, systems should consider how they can use apprenticeships to support staff recruitment and retention. We recommend that the apprenticeship levy held by trusts is used where appropriate.
A nationally led safe and sustainable CDC international recruitment programme has been developed to supply a trained and skilled workforce to UK CDCs. The programme provides onboarding, clinical supervision and mentorship resourcing to support the retention of international staff. Systems are encouraged to engage in the programme to ensure that they have packages that facilitate joining the UK workforce, meet recruitment timescales and offer contracts with a minimum 2-year tenure to support retention.
Health inequalities
CDCs will help reduce the health inequalities across local populations that are driven by unwarranted variation in referrals, access, uptake, experience and outcomes of diagnostic provision. In implementing CDCs, systems will have fully and thoroughly evaluated where these should be sited with consideration of accessibility and addressing inequality and deprivation.
Systems are now asked to ensure that their CDC evaluations assess impact on population health and local health referrals, and that they use this data to inform next steps in CDC development.
Systems should ensure that CDCs operate in an inclusive way; for example, catering for patients with disabilities, neurodiversity and those who need interpreters.
Data, reporting and evaluation
CDCs should record all activity and report this weekly (by 5pm every Wednesday) via the CDC portal. CDCs must commit to aligning their funded activity plans to their reporting each week using the review and management of performance process to communicate any underperformance, delays or data lapses in a timely manner.
CDCs must report all activity under their designated ODS code onto SUS as they move to a national contracting and commissioning framework, anticipated to be in 2025/26.
There is an expectation that utilisation metrics will be monitored, reviewed and actioned to meet agreed productivity levels as part of effective management of services.
CDCs must also comply with any other agreed reporting requirements, including reporting against all nationally agreed metrics and key performance indicators (KPIs) for the CDC Programme (Appendix C). All CDCs are expected to report monthly on DM01 and separately from their acute site submissions.
Systems must evaluate their CDCs at regular intervals (at least quarterly) to understand their performance against the stated aims of the programme, and from this identify areas for improvement.
Patient and staff experience
Once operational, CDCs are expected to join the Experienced Based Design (EBD) Programme rollout and should run an annual EBD survey with patients and staff and use the feedback to improve services in the CDC. Revenue funding has been made available to support the required improvements identified through this work.
Systems should review qualitative data from EBD to set the health inequalities agenda in terms of patient experience and reach into targeted groups.
NHS Elect is supporting this rollout and further information is available on the CDC FutureNHS page.
Developing clinical pathways for CDCs
All CDCs must offer the opportunity to optimise clinical pathways, ensuring that they are efficient and effective and deliver the right tests to support early diagnosis and inform the advice and treatment for each patient. Appendix B outlines the requirements for the patient journey through the CDC.
As CDCs further operationalise, systems should now focus on 3 aspects of pathway design:
- Innovation – how can pathways be optimised through harnessing digital diagnostics, new ways of working and other innovations?
- Experience – how can pathways be designed to optimise patient experience by offering a connected and personalised experience through a range of quality diagnostic services?
- Efficiency – how can pathways be designed to reduce duplication and length of time to diagnosis through maximising use of infrastructure, effective use of diagnostic equipment, and reducing overall demand with clinical support tools and technology to share information?
A national list of priority pathways to be commenced will be published annually and we will work with systems to understand how this will be completed and what resources are required to deliver on this in year. This will be monitored regionally and nationally as a key component of CDC success.
Pathways should be co-developed with patients and staff and relevant patient charities should be engaged.
Funding is in place for CDCs to develop their pathways. Those awarded funding are asked to commit to participating in communities of practice to share learning, providing quarterly updates and a summary report, and reporting against agreed metrics from point of pathway go live, in line with the CDC Programme requirements.
4. Next steps and support
CDCs are a key part of transforming the way we provide elective care and are already providing a growing proportion of elective diagnostic provision across systems and regions.
- all systems have been supported to have in place at least one standard or large CDC, and in 2024/25 we will monitor and evaluate their impact against activity plans and stated aims
- we will focus on refining the revenue allocation process and support delivery of the maximum amount of activity to meet system performance needs. Together NHS England and systems will monitor and track delivery against allocated capital and revenue budgets and share best practice
We will evaluate programme delivery and lessons learnt and use this information to shape next steps and identify future priorities for CDCs.
Support is available from NHS England national and regional programme teams as well as the materials and information on the CDC FutureNHS page. Systems are advised to access support where needed and asked to feedback via england.cdcprogramme@nhs.net on what best supports them in achieving their ambition. We encourage them to share their learning and best practice via local, regional and national routes including FutureNHS.
Appendix A: Minimum activity levels and utilisation rates for CDC sites
Imaging
Modalities | 5-day working week | 6-day working week | 7-day working week |
|---|---|---|---|
|
MRI – static |
110 |
132 |
154 |
|
MRI – mobile |
110 |
132 |
154 |
|
CT – static |
165 |
198 |
231 |
|
CT – mobile |
165 |
198 |
231 |
|
X-ray |
315 |
378 |
441 |
|
Ultrasound |
110 |
132 |
154 |
Endoscopy
Royal colleges and professional societies relating to endoscopy are reviewing the national guidance on the number of procedure points for the varying endoscopy procedures to be booked on an endoscopy list. This guidance will be updated once national policy is confirmed.
Physiological science
Respiratory investigations | 5-day week | 6-day week | 7-day week |
|---|---|---|---|
|
Dynamic assessments (spirometry) (± BD) |
80 |
96 |
112 |
|
FeNO |
150 |
180 |
210 |
|
Full lung function |
50 |
60 |
70 |
|
Blood gases (capillary) |
16 |
19 |
22 |
|
Simple exercise (field test) |
10 |
12 |
14 |
|
Home diagnostic sleep study |
25 |
30 |
35 |
Cardiac investigation | 5-day week | 6-day week | 7-day week |
|---|---|---|---|
|
12-lead ECG |
150 |
180 |
210 |
|
Ambulatory ECG monitoring (for example, Holter, patient activated) |
30 |
36 |
42 |
|
Echo |
30 |
36 |
42 |
|
Pathology (taking blood tests) |
330 |
396 |
462 |
Optimal rates
- CT: 3–4 scans per hour
- MRI: 2–3 scans per hour
- NOUS: 3 scans per hour
- Echo: 1 scan per 45 min, including reporting
- Endoscopy: 85% of planned endoscopy lists taking place
Appendix B: Requirements for patient journey through the CDC
- Receive and process referrals from primary, community and secondary care: This should include the development of clear referral criteria for an agreed set of pathways and/or tests. Referrals should be considered from primary care, appropriately trained first contact practitioners, NHS screening services, paramedics, community services (such as rapid response teams), urgent treatment centres, NHS 111, emergency departments and specialist secondary care providers. Self-referral for agreed patient groups could be included where systems feel this is helpful.
- Booking and preparation: Book patients in for a co-ordinated set of tests. CDCs must provide patients in advance of attending with information explaining the diagnostic tests they will have and anything they need to do to prepare for these tests. Systems should consider implementing multiple methods for booking appointments to improve accessibility.
- Consultation: Provide virtual or face-to-face support to a consultant specialist where this supports efficient patient pathways.
- Co-ordinated testing: Carry out a full range of diagnostic tests for patients in as few visits/locations as possible.
- Reporting: Report the results in a timely way to the referrer. CDCs must have the appropriate digital infrastructure and connectivity in place to share information and report on the outcomes of diagnostic tests and procedures, to inform onward clinical care. Wherever possible, reporting should offer the GP advice and guidance on a management plan for the patient.
- Diagnosis and prescription: Communicate a diagnosis or treatment plan direct to the patient where required and appropriate, and where communication is to systems ensure that appropriate wraparound patient support is available.
- Onward referral: Provide onward referral if needed as part of the patient pathway. This should be within the scope of agreed protocols and recognise that clinical responsibility for the patient journey remains with the original referring clinician. CDCs must keep the referring clinician informed where onward referrals are made.
Appendix C: CDC benefits matrix metrics
Benefit | Metric | |
|---|---|---|
|
1 |
Improved population health outcomes |
Increase % of GPs with direct diagnostic referrals into a CDC electronically |
|
2 |
Improved population health outcomes |
Referral to scanning time for urgent referrals at a CDC is less than 14 days |
|
3 |
Improved population health outcomes |
Reduced time from referral to diagnostic report complete and delivered to GP/secondary care for funded pilot pathway sites |
|
4 |
Increase diagnostic capacity |
Number of core tests performed in a CDC: CT, MRI, echo and NOUS |
|
5 |
Increase diagnostic capacity |
Total number of CDC sites operational and delivering activity increases year on year of programme |
|
6 |
Improved productivity and efficiency |
Actual activity delivered against planned capacity |
|
7 |
Contributing to reducing health inequalities |
Number of CDCs located in areas of deprivation: number of tests mapped against depreciation scores |
|
8 |
Deliver better and more personalised service |
Review of touchpoints for staff and patients detailing their experience of CDCs and whether this is positive or negative, and what learning comes from the narrative |
Publication reference: PRN00953

