1. Introduction
1. There are two seasonal influenza enhanced service programmes in 2024/25 that practices can participate in:
i) The Enhanced service specification: seasonal influenza vaccination programme 2024/25 (“Seasonal Influenza ES”) enables practices to administer influenza vaccinations to eligible adult and at-risk patients as follows:
a) where practices individually administer influenza vaccinations to eligible patients registered with the practice or using the Immediately Necessary Treatment status for specific eligible patients not registered with the practice; and/or
b) as part of an Influenza collaboration agreement; and/or
c) as part of a PCN grouping COVID-19 Collaboration Agreement where the influenza vaccination is co-administered with a COVID-19 vaccination or synergistically administered alongside a COVID-19 vaccination by a practice’s PCN grouping (where synergistic refers to influenza-only vaccination clinics run alongside COVID-19 vaccination clinics); and in accordance with the Seasonal Influenza ES.
ii) The Enhanced service specification: childhood influenza vaccination programme 2024/25 (“Childhood Seasonal Influenza ES”) enables practices to administer influenza vaccinations to eligible 2 and 3 years olds as follows:
a) as part of the usual annual arrangements where practices individually administer the influenza vaccinations to eligible patients registered with the practice; and/or
b) as part of an Influenza Collaboration Agreement; and in accordance with the Childhood Influenza ES
2. Practices wishing to participate in the Seasonal Influenza ES and Childhood Influenza ES must indicate their willingness to do so to their commissioner before 23:59 on 30 June 2024. Where practices also wish to participate in the Enhanced Service Specification: COVID-19 Vaccination Programme 1 September 2024 to 31 March 2026 (“COVID-19 ES”) then the practice must have signed up to the Seasonal Influenza ES before 23.59 on 30 June 2024.
3. This guidance provides information on the process for recording influenza vaccination events, claims for the Item of Service (IoS) fees and vaccine reimbursement payments depending upon whether the influenza vaccination event is:
i) administered individually by a practice to eligible registered or unregistered patients;
ii) as part of an Influenza Collaboration Agreement; or
iii) as part of a PCN grouping COVID-19 Collaboration Agreement.
4. It is important that practices follow this guidance to avoid payment duplications or recording errors, and overpayment recovery.
5. Some practices may have access to both a GP IT Clinical System and a Point of Care (PoC) system. For each influenza vaccination event, practices must only record in one, not both, of these systems. The system to be used is dependent on whether the vaccination event was administered by the practice individually, under an Influenza Collaboration Agreement, co-administered or synergistically delivered as part of a PCN grouping under a COVID-19 Collaboration Agreement.
i) Vaccination events delivered individually by practices or as part of an Influenza Collaboration must be recorded in GP IT clinical system and not their PCN grouping PoC system.
ii) Vaccination events co-administered with COVID-19 as part of a PCN grouping must be recorded in the PoC system using the co-administration template and not their practices’ GP IT clinical systems.
iii) Vaccination events synergistically delivered alongside COVID-19 as part of a PCN grouping must either be recorded in the PoC system or the practices’ GP IT clinical system, not both. Practices in PCN Groupings must therefore agree which system they will all record synergistically delivered influenza vaccinations administered as part of a PCN grouping and use only that system.
6. Single system recording is imperative to avoid duplication in clinical records and payment. Such consequences must be avoided so that practices can maintain accurate clinical records and be paid correctly for each vaccination event undertaken.
7. NHS England has commissioned the NHS Business Services Authority (NHSBSA) to support post payment verification and data quality validation for influenza vaccination claims. Claims made by practices will be subject to review. NHSE will undertake data quality validation during the same period; this will provide assurance around accuracy of clinical records.
2. Individual practice administering influenza vaccinations
2.1 Influenza vaccinations administered to eligible patients registered with the practice
8. Practices must record influenza vaccinations administered to eligible registered patients as per the usual process within their clinical systems. The General Practice Extraction Service (GPES) will collect the relevant clinical information each month, using the defined clinical codes within the GPES business rules, on the number of patients on the practice’s registered list and who are recorded as being vaccinated against influenza during the relevant reporting period. This information is passed to the Calculating Quality Reporting Service (CQRS) accordingly and the relevant IoS payments will be made to the practice monthly.
9. To enable CQRS to calculate the monthly payment achievement, practices are required to confirm participation in the Seasonal Influenza and Childhood ES within CQRS by 31 July 2024 unless otherwise notified by the commissioner (this CQRS participation date is separate to the sign-up deadlines for participating in the influenza services, which is outlined in paragraph 2). Practices confirming participation via CQRS after this date may not have data available for CQRS for the full period of the enhanced
10. Each GPES data collection will capture data for all payment and management information counts and report on activities from the start of the reporting period, e.g. 1 September to the end of the relevant reporting Data will be collected one month in arrears, e.g. data for September will be collected in October. GPES provides the monthly counts to CQRS.
11. If automated collection via GPES is not available for any reason, practices would be required to manually input data into CQRS, until such time as GPES becomes available again (as communicated via NHSE). For information on how to manually enter data into CQRS, practices should refer to the CSU Collaborative website.
12. Practices must only use the relevant clinical codes included in the supporting Business Rules and should re-code patients where necessary, for example if there is an error in coding. This will allow CQRS to calculate payment and for the Commissioner (NHSE), and/or NHSBSA acting on the Commissioners behalf, to audit payment and service delivery. Practices should refer to the supporting Business rules for the most up-to- date information on management counts and clinical codes.
13. Where there is an automated data collection, via GP IT clinical systems, there is a five- day period following the month end to allow practices to record the previous month’s activity before the collection occurs. Activity submitted after the collection period is closed will not be collected and recorded on Practices must ensure all activity is recorded by the cut-off date to ensure payment.
14. The GPES extract for the Seasonal Influenza ES has been updated to phase the cohorts in line with the relevant programme start dates. Payment extracts for children (aged 6 months to 17 years) in clinical risk groups and for pregnant women will start from 1 September Payment extracts for adults aged 18 and over will start from 3 October 2024.
2.2 Influenza vaccinations to eligible patients that are not registered with the practice
15. For 2024/25, practices can vaccinate specific patients that are not registered with them, namely:
i) frontline workers in social care settings employed by the following types of social care providers without employer led occupational health schemes:
a) a registered residential care or nursing home, or
b) a voluntary managed hospice provider
ii) those patients living in long-stay facilities, nursing homes, other long-stay health or social care facilities; and
iii) locum GPs
16. Practices should first register the patient within their existing GP IT clinical system using the Immediately Necessary Treatment (INT) registration status.
17. The use of INT registration status provides a mechanism to record the vaccination event given to eligible patients that are unregistered with the practice under the terms of the Seasonal Influenza ES specification. It is being used solely for administrative purposes in order to create a patient record for the influenza vaccination and enable payment accordingly. It does not oblige the vaccinating practice to provide other types of INT care to those patients.
18. Practices should then code the influenza vaccination event, using clinical codes as per the normal process for registered patients, as follows:
i) using the clinical codes only to capture the vaccination event for those patients living in long-stay facilities, nursing homes, other long-stay health or social care facilities; and
ii) using the clinical codes AND ‘needs influenza’ code for eligible unregistered health and social care staff (i.e. frontline workers as per paragraph 15.i. and locum GPs).
19. The vaccination event record will then flow via the Data Processing Service (DPS) to the patient’s registered practice, where it will be recognised and therefore excluded from the standard GPES extract For practices using SystmOne (TPP), EMIS web (EMIS) and Vision 3 (Cegedim) clinical systems the record of the influenza vaccination event will be automatically transferred from the practice that administers the vaccination on an INT basis to the patient’s registered practice clinical system via the DPS. No further action is required by the vaccinating practice or the patient’s registered practice apart from where there are abnormal conditions which would require action. However, the INT registration status is not available in EMIS web PCN hub, Vision Anywhere or Vision 360 system, so influenza vaccinations administered in respect of patients registered with another practice should not be recorded within these systems and instead in EMIS web, SystmOne or Vision 3.
20. Vaccination events recorded within the GP clinical system in line with the above process for the period from 1 September 2024 to 31 March 2025 will be extracted monthly in the seasonal influenza service GPES Payment of the IoS fee will be made to the practice that has administered the vaccination on a monthly basis.
21. If a practice has recorded an unregistered patient’s influenza vaccination using the temporary resident (TR) status, instead of the INT status, then the practice should not change their If a practice were to re-record the vaccination again using the INT status this would create a duplicate event for the same patient. Where a practice has recorded a patient as a temporary resident then the practice will be required to submit a manual claim to their local commissioner for those who have been registered as a temporary resident.
22. Practices must not use the INT coding for the administration of vaccinations to eligible frontline patient-facing primary care staff (except for GP locums) as set out in section 7.
23. Practices should claim reimbursement for the cost of influenza vaccines administered to patients not registered with the practice in the usual way by submitting the FP34PD form (or FP34D form for dispensing contractors) to the NHSBSA.
24. Annex A provides supplier guides on recording influenza vaccinations using the INT registration status.
3. Influenza vaccinations administered as part of an Influenza Collaboration
25. Practices may, under the terms of the Seasonal Influenza ES and the Childhood Influenza ES, collaborate to administer influenza vaccinations to their patients as part of an Influenza Collaboration. Practices collaborating under an Influenza Collaboration will be deemed a temporary single medical practice for the purpose of regulation 3(5), (8) and (9) of the Human Medicines Regulations 2012 (as amended). Regardless of whether practices are collaborating as part of an established Primary Care Network (PCN) or not, all practices in the collaboration will be required to sign up to an Influenza Collaboration Agreement where the practices wish to collaborate to deliver influenza vaccinations.
26. In this section, references to ‘collaborating practices’ refers to practices providing influenza vaccinations under an Influenza Collaboration.
27. Patients that are eligible to have an influenza vaccination from collaborating practices, will be either:
i) an eligible patient whose name appears in the registered patient list of one of the collaborating practices under both the Seasonal Influenza ES and Childhood Influenza ES, or
ii) eligible unregistered patients under the Seasonal Influenza ES (for example patients living in long-stay residential care or nursing homes).
28. Practices will be required to agree and set out within their Influenza Collaboration Agreement the designated site(s) at which their patients will be able to access an influenza vaccination under an Influenza Collaboration. As such, any eligible registered or unregistered patients will only be able to access influenza vaccinations from these designated site(s) as part of an Influenza Collaboration and cannot receive an influenza vaccination by just attending any of the other collaborating practices where they are not the designated site(s).
29. A proportion of influenza vaccine may be supplied by collaborating practices to their temporary single medical practice under Regulation 19(4A) of the Human Medicines Regulations 2012 (as amended). Collaborating practices will be required to set out within the Influenza ES Vaccination Collaboration Agreement:
a) the vaccine sharing arrangements, including details of the proportions of the influenza vaccine shared;
b) how collaborating practices will govern the supplied proportion of influenza vaccine; and
c) how collaborating practices will claim reimbursement for any administered influenza vaccine supplied and any personal administration (PA) fee (where eligible).
30. Please refer to the collaboration section(s) and Annex B of the Seasonal Influenza ES for full details.
31. Some example scenarios to illustrate how the single temporary medical practice may administer influenza vaccines when operating under an Influenza Collaboration are provided This list is not exhaustive but illustrates the key requirements in terms of eligible patients, approved site and vaccine provision.
i) Example 1: Practices A, B, C and D enter into an Influenza Collaboration. Under the terms of the Influenza Collaboration Agreement, Practice B is the approved site for the administration of influenza vaccinations on Tuesdays and it has been agreed that Practice C will provide some of their influenza vaccine to Practice B to use in the Tuesday influenza A 69-year-old registered patient of Practice A makes an appointment at the approved Practice B site of the Influenza Collaboration, on a Tuesday when staff from Practice B are administering influenza vaccines. This patient can receive the influenza vaccine (procured by Practice C and supplied to the single temporary medical practice, administered by Practice B staff acting on behalf of the single temporary medical practice, whilst being a registered patient of Practice A which is a member of the single temporary medical practice under the Influenza Collaboration Agreement).
ii) Example 2: Practices A, B, C and D enter into an Influenza Collaboration. A pregnant woman who is a registered patient of Practice B walks into the surgery of Practice C and asks for an influenza vaccine. This patient cannot receive the influenza vaccine as she is not a registered patient of Practice C and she has attended at Practice C which is not the approved site of the Influenza Collaboration. She has not attended at the single temporary medical practice.
iii) Example 3: Practice A is due to vaccinate patients at a long stay residential care home next Friday. Patient 1 and Patient 2 are both residents at the care home. Patient 1 tells patient 2 that her practice, Practice A, is providing the influenza vaccinations. Patient 2 is not registered with Practice A, but is registered with practice B. Patient 2 asks for Practice A to provide his influenza vaccine and Practice A can do this. Patient 2 is registered at Practice B but has chosen to receive his vaccine from Practice A at the same time as Patient Practice A can administer this influenza vaccination to Patient 2 outside the Influenza Collaboration and under Practice A’s influenza enhanced service terms to vaccinate eligible unregistered patients.
32. Where individual practices are also offering separate additional clinics or opportunistic influenza vaccinations outside the Influenza Collaboration at their usual practice site(s), their registered patients or specific eligible unregistered patients will still be able to access influenza vaccinations. In these circumstances, this is outside of an Influenza Collaboration that the practice may also be participating in.
3.1 Recording influenza vaccinations and claiming payments as part of an Influenza Collaboration
33. Practices collaborating under an Influenza Collaboration must record influenza vaccinations administered as follows:
i) to eligible registered patients as per the usual process within their registered practices’ clinical systems as set out in section 2.1 of this guidance; or
ii) using the INT registration process for eligible unregistered patients within the clinical system of the practice administering the vaccination as set out in section 2.2 of this guidance; and in accordance with the relevant service specification.
34. For eligible registered patients, the IoS payments will be paid to the patient’s registered practice. For eligible unregistered patients, the IoS payment will be paid to the practice that administered the influenza vaccination and recorded it using the INT registration status. Collaborating practices will need to agree how any received IoS payments in respect of the patients to whom they administer influenza vaccinations as a collaboration will be allocated.
35. Claims should be made in accordance with the relevant seasonal influenza enhanced service specification. See section 6 of this guidance for further details on claiming influenza vaccine costs and the personal administration fee.
4. Influenza vaccinations administered as part of a COVID-19 Collaboration
36. Practices co-administering or synergistically administering influenza vaccinations as part of a PCN grouping COVID-19 Collaboration Agreement must do so in accordance with the COVID-19 ES and the Seasonal Influenza ES. A template COVID- 19 Collaboration Agreement is available to support co-administration and synergistic delivery of influenza vaccinations with COVID-19 vaccinations.
37. Co-administered influenza vaccination events administered as part of a COVID-19 Collaboration Agreement must be recorded in the PCN grouping’s PoC System and the subsequent IoS payments will be made to the nominated host practice. Synergistically delivered influenza vaccinations must be recorded in either the PCN grouping’s PoC System or the individual PCN grouping practices GP IT clinical systems, but not both.
38. Reimbursement claims for the influenza vaccines administered as part of a PCN grouping arrangement are to be made by individual practices in accordance with the General Medical Services Statement of Financial Entitlements (SFE) – see section 6 of this guidance. PCN groupings (or PCNs) cannot claim flu vaccine reimbursement.
39. The 2024/25 Financial and Payments Guidance for COVID-19 Vaccination Programme on recording co-administered and synergistically delivered influenza vaccinations between September 2024 and March 2025 and the Seasonal Influenza Vaccination Programme will be available in due course.
5. Limitation period for claiming an IoS fee
40. Claims for activity related to the administration of influenza vaccinations only must be submitted on the relevant system as soon as possible. Practices must validate and submit a claim to the Commissioner for payment within three months of the date of the administration of the completing dose of the vaccine as outlined in Section 11 Payment and Validation of the Seasonal Influenza ES.
41. Where an influenza vaccination is co-administered with a COVID-19 vaccination, then claims must be submitted within three months of the date of the administration of the completing dose of the vaccine.
6. Reimbursement of the influenza vaccine costs and personal administration fee
42. Practices will continue to claim for locally procured influenza vaccine costs and personal administration (PA) fees in accordance with the General Medical Services Statement of Financial Entitlements (SFE) and using the established systems for doing so. For the avoidance of doubt, influenza vaccine reimbursement claims are to be submitted by individual practices only and not by a PCN grouping/PCN.
43. Collaborating practices administering influenza vaccinations as part of either an Influenza Collaboration or COVID-19 Collaboration (i.e. co-administration or synergistic delivery of influenza vaccinations with COVID-19 vaccinations) will need to agree between them how the collaborating practices will individually claim reimbursement, and any associated PA fee, for any administered influenza vaccines they have
44. Collaborating Practices will need to agree how they will manage the influenza vaccine supplied by each of their collaborating practices to allow individual practices to claim accordingly to the agreement they reach, as per the usual process each practices applies every year and using the established systems to do so. To support this, the relevant schedule should be completed in either the:
i) Influenza Collaboration Agreement, if in respect of an Influenza Collaboration; or
ii) COVID-19 Collaboration Agreement, if in respect of a PCN grouping COVID-19
45 Collaborating practices and PCN Groupings must keep clear and up to date records on the administration and movement of influenza vaccines to support any claims made for IoS fees, reimbursement, or PA fees. NHSBSA website includes some of the details that may be requested as part of post payment verification either during or following the 2024/25 influenza season.
46. Practices will not be reimbursed for any influenza vaccine that has been centrally supplied.
7. Influenza vaccinations for frontline primary care staff
47. Practices may under the 2024/25 Seasonal Influenza ES offer their frontline patient- facing staff an influenza vaccination. This forms part of employer occupational health responsibilities and can be provided either by the employing practice or under other arrangements, for example through an occupational health provider or influenza voucher scheme with a community pharmacy.
48. Where eligible frontline patient-facing staff are administered an influenza vaccination by their employing practice, the practice will not be eligible for reimbursement of the influenza vaccine cost nor an IoS payment. This is with the exception of where the eligible frontline patient-facing staff member is:
i) eligible under the NHS influenza programme due to age or clinical risk AND is a registered patient at their employing practice, or
ii) a GP locum
49. With the exception of a GP locum as set out in section 2 of this guidance, practices must not use the INT status to record influenza vaccinations administered to their eligible frontline patient-facing staff.
Annex A: Immediate Necessary Treatment (INT) system specific supplier guides
A1. Cegedim (Vision 3):
To record the vaccination of immediately necessary patients
1. Register the patient using the INT registration See Adding an Immediately Necessary Treatment Patient for more information.
Note – the INT registration status is being used for administrative purposes in order to create a patient record for the purposes of influenza vaccination only. This does not obligate the vaccinating practice to provide INT owing to an accident or an emergency to patients not registered with the practice that they are administering an influenza vaccination to.
2. Record the vaccination event as you would do normally for registered, see Delivering the vaccine for more information.
Information – practices should ensure that vaccinations of these patients are recorded in line with this guidance to ensure they receive the appropriate payment. Practices should not administer influenza vaccinations to any patients that are not registered with the practice outside of the specified guidance.
Payments for immediately necessary patients
Vaccinations recorded in line with the above process are extracted via a monthly GPES extraction and payments are made to practices monthly.
Note – practices should claim reimbursement for influenza vaccines administered to patients not registered with the practice in the usual way by submitting FP34 forms to the NHS Business Services Authority (NHSBSA).
A2. EMIS (EMIS web)
Seasonal influenza – patients not registered at your organisation
The Seasonal Influenza Vaccination Programme 2024/25 enables practices to vaccinate specific patients that are not registered.
Recording vaccinations of immediately necessary patients
To do this, patients need to be registered in EMIS Web as INT as this allows their details to appear on the system and the practice can claim payment for the vaccine issued.
Please note that the INT registration status is being used for administrative purposes in order to create a patient record for the purposes of influenza vaccination only and does not obligate the vaccinating practice to provide INT owing to an accident or an emergency to patients not registered with the practice that they are administering an influenza vaccination to.
Practices should not administer influenza vaccinations to any patients that are not registered with the practice outside of the groups specified within the Seasonal Influenza ES.
Registering a patient as immediately necessary
Patients registered to the practice with the status of immediately necessary will expire after 14 days and become inactive.
Access registration
Click the EMIS icon (top left), point to registration, and then select registration. The registration screen is displayed.
Click add patient and select immediately necessary as the patient type.
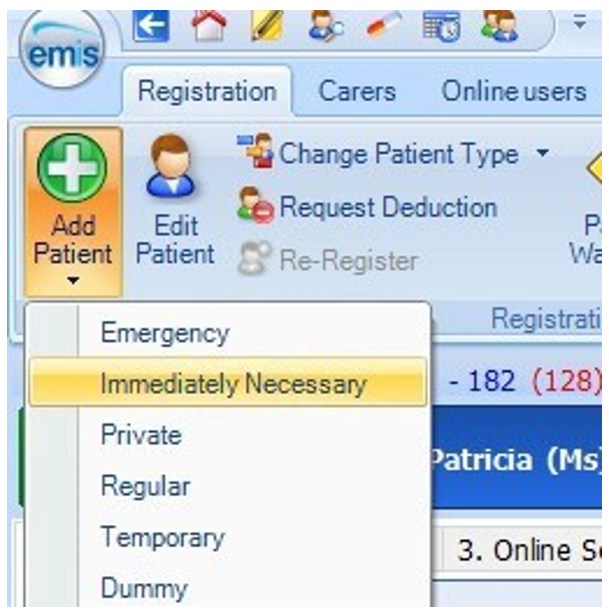
Complete the patient trace screen to find the patient.
You must complete all mandatory fields (marked with a red asterisk *); if you don’t complete a mandatory field (except NHS number), a warning icon is displayed.
Click find; the system will show that no patient can be found. Select yes to continue adding the patient.
Fill out the details within the add patient screens as necessary.
The only mandatory fields (marked with a red asterisk *) needed are within the patient details and primary care sections of the add immediately necessary patient screen.
Recording the vaccination event
Follow your practice procedure or protocol for administering the vaccine. Resources to help get you started with the 2024/25 influenza season can be found here: Login – Customer Support (emisnow.com)
Transfer of records to the patient’s registered practice
For practices using EMIS Web clinical systems the record of the vaccination event will be automatically transferred to the patient’s registered GP practice clinical system via NHS Digital’s Data Processing Service.
No further action is required by the vaccinating practice or the patient’s registered practice provided the receiving organisation is enabled to autofile influenza vaccines.
When receiving updates into your practice, if an influenza code already exists on the patient’s record, EMIS Web will provisionally file the influenza vaccine code contained in the Digital Medicine message to the patient’s record. This entry will be displayed as [Provisional] within a Consultation. This could be due to one of the following:
- an existing influenza vaccine within the current influenza season
- a future diary entry for an influenza vaccine
- a record of an influenza vaccine allergy
A workflow task will be created for a user to review the documentation received in the task and add additional information if required, before filing to complete the task.
Patients without a registered practice
If the patient does not have a registered practice, the vaccination event will flow to the Data Processing Service only.
Payment Process
Vaccination events recorded within the GP clinical system, in line with the above process, will be extracted via a monthly GPES extraction and payments made to practices monthly.
GPES will identify the patient’s immediately necessary treatment registration status and
Influenza vaccine code to claim payments.
A3. SystmOne (TPP)
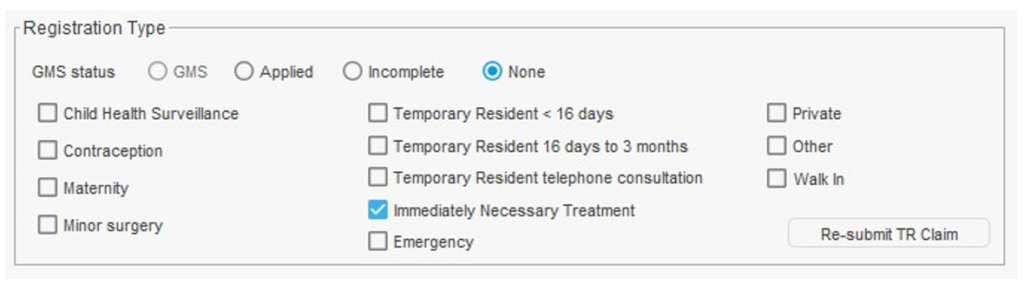
Using the immediate necessary treatment registration type in SystmOne
To register a patient for immediate necessary treatment (INT), during the registration process you should select the GMS Status of ‘None’ and then tick the box for Immediate Necessary Treatment.
Figure 1. Registering a patient using the INT registration option
Annex B: frequently asked questions (FAQs)
These FAQs have been developed to provide additional information to support practice delivery of the 2024/25 annual influenza programme. Practices should read this section alongside the 2024/25 Annual Flu Letter, the amended Annual Flu Letter, the two published Seasonal Influenza Vaccination Programme Enhanced Service specifications and the Influenza Collaboration Agreement.
Eligible cohorts
2. What cohort changes have been made to the adult Seasonal Influenza Vaccination Programme Enhanced Service Specification for 2024/25?
The Seasonal Influenza ES includes the list of eligible patients which remain unchanged for 2024/25.
The provision of influenza vaccinations for primary care staff, like other frontline healthcare staff, continues to be an employer’s responsibility.
3. What is the reason for the change in start date for the seasonal influenza programme?
Evidence suggests that influenza vaccine effectiveness can wane over time in adults, hence JCVI have advised moving the start of the programme to October, in line with the announced and authorised start date, for most adults as it is preferable to vaccinate individuals closer to the time when the influenza season commonly begins. Circulation of the influenza virus usually peaks in December or January, therefore vaccinating most individuals in October and November provides optimal protection during the highest risk period.
The start date has not changed for pregnant women due to the vaccination protecting babies in the first few months of their lives. It is not expected that pregnant women will lose protection as rapidly as the older population or other clinical risk groups, therefore starting vaccination earlier will still offer protection to the women themselves whilst also ensuring as many newborn babies as possible are protected during the influenza season.
Protection from the vaccines for children lasts longer, and starting earlier for children may help to reduce transmission to the wider population, therefore there is no change to the start date for this cohort.
The UKHSA have produced a leaflet for patients explaining when they will be able to get their flu vaccine. This resource is available for practices here: When can I get my flu vaccine ? – Health Publications
4. When will practices be advised of the start date for the seasonal influenza (adult at-risk) programme?
NHS England announced on 13 June 2024 that the authorised start date for the 2024/25 seasonal influenza adult (at-risk) vaccination programme is 3 October 2024.
5. If adults at risk are not being vaccinated until October, when are practices required to vaccinate pregnant women?
Pregnant women are an exception to the advice on the later start date and should be vaccinated from 1 September 2024. See question 3 for details on the rationale for the different start dates.
6. What if a patient requires an influenza vaccination before the announced and authorised start dates of the programme? Will practices be able to offer an influenza vaccination in September and still get an item of service payment?
Practices may in exceptional clinical circumstances, following a clinical assessment, vaccinate individuals for whom it would be better not to delay influenza vaccination until the announced and authorised start date. It is anticipated this would apply to a small number of individuals. The Seasonal Influenza ES contractual start date is from the 15 August to allow practices to be covered under the Clinical Negligence Scheme for General Practice (CNSGP) and to be paid for vaccinations administered in these exceptional clinical circumstances.
7. What are the payment processes if a patient requires an influenza vaccination before the announced and authorised start date of the programme?
Practices will be required to submit a manual claim for any administered influenza vaccinations administered in exceptional clinical circumstances as outlined in FAQ 6 and in either of the following time periods:
- a child aged 6 months to 17 years or a pregnant adult (who are at-risk) between 15 August 2024 and 31 August 2024, and
- an adult aged 65 years or over or aged 18 to 64 years in a clinical risk group between 15 August 2024 and 2 October 2024.
The claims should be submitted using the same process that would normally apply when submitting a manual claim for commissioner approval and in accordance with the Seasonal Influenza ES.
8. Can those patients who are 64 years but turn 65 years before 31 March 2025 be vaccinated from 3 October 2024?
Yes. However, vaccination of those patients who are 65 years and over should be clinically prioritised over those healthy patients aged 64 turning 65 years by 31 March 2025. Practices should vaccinate this cohort of patients with the recommended vaccine as specified within the Seasonal Influenza ES and the statement of amendments to the annual flu letter published 12 June 2024.
9. Can GPs use QIVc for healthy 64 year olds that turn 65 years before 31 March 2025 even if they have aQIV or QIV-HD in stock
The Seasonal Influenza ES and Flu Letter advises that the recommended vaccine is aQIV to be offered ‘off label’ for those turning 65 before 31 March 2025. QIVc may be offered only when every attempt to use aQIV or QIV-HD has been exhausted. Evidence of this may be requested by the commissioner before reimbursement is agreed. Details of vaccines to used for the 2024/25 influenza season can be found in the Seasonal Influenza ES and the flu letter
10. How can eligible individuals who are not currently registered with a practice access an influenza vaccination this season?
There may be a small number of patients who are not currently registered with a practice who are eligible for an NHS influenza vaccination and who may self-present at any practice to request an influenza vaccination. Whilst an NHS number or registration at a practice is not essential for vaccination, practices should encourage the patient to register with a practice, particularly for some vulnerable groups such as rough sleepers; homeless is not a criterion in itself to qualify for an influenza vaccine but individuals may have other health issues that make them eligible and would make them especially vulnerable to influenza.
Any eligible adult patient can access a vaccine at a community pharmacy, regardless of whether they are registered with a practice.
11. What is the procedure for NHS primary care frontline patient-facing staff to receive an influenza vaccination this season?
General practice are expected support the vaccination of their frontline patient-facing staff. As with the 2023/24 season general practice can provide flu vaccinations to their own eligible frontline patient-facing staff in accordance with the Seasonal Influenza ES and are eligible for cover under the Clinical Negligence Scheme for General Practice (CNSGP) – see section B.4. Practices, however, are not eligible to claim for the item of service fee for the administration of the influenza vaccine, reimbursement of the vaccine cost or personal administration fees relevant to these vaccinations.
B2. Vaccines
B2.1 Alternative vaccines for children
12. What are the arrangements for offering an alternative vaccine for children where there is an objection to the porcine gelatine content in the vaccine?
Practices can offer an alternative injectable vaccine (QIVc) to healthy children if requested by the parent or guardian who objected to LAIV on the grounds of porcine gelatine content. Practices may offer this routinely from the start of the season where applicable.
B3. Sub-contracting
13. Can practices sub-contract arrangements to administer influenza vaccinations?
Practices administering influenza vaccinations must do so in accordance with the Seasonal Influenza Enhanced Service Specifications. A practice may sub-contract administration of influenza vaccinations in accordance with the relevant Regulations or Directions and their core GMS/PMS/APMS contract and must have all relevant consideration for medicines handling regulations, governance and standards.
14. How should practices record vaccinations under sub-contracting arrangements?
Practices and their sub-contractor must ensure that appropriate data management processes are in place which must include the recording of the administration of influenza vaccinations to ensure that payment can be made in accordance with the Seasonal Influenza Service Specification or in accordance with any alternative written agreement between the Practice and the Commissioner (NHS England).
15. Can practices administer childhood influenza vaccinations in another setting?
The Childhood seasonal influenza enhanced service specification for 2 and 3 year olds includes provision for practices to administer childhood influenza vaccinations via sub- contracting arrangements in certain circumstances. Additionally, practices may work together locally under an Influenza collaboration agreement to administer influenza vaccinations to 2 and 3 year olds.
B4. Indemnity
16. Will practices be covered by CNSGP when providing influenza vaccinations to eligible patients that are not registered with their practice?
Yes. The Seasonal Influenza ES outlines the eligible cohorts that practices can vaccinate regardless of whether the person is registered with the practice. If the practice provides influenza vaccinations under the terms of the Seasonal Influenza ES to eligible unregistered patients then any clinical negligence that may arise through this activity would fall within the scope of the CNSGP. This activity falls within the scope of the CNSGP scheme as the service being provided under the terms of the Seasonal Influenza ES and therefore under a primary medical services contract (GMS, PMS, APMs or Schedule 2L provisions under NHS Standard Contract).
17. Will practices be covered by the CNSGP when providing influenza vaccinations to members of their practice staff?
The Clinical Negligence Scheme for General Practice does cover influenza vaccinations for primary care staff provided by their employer under occupational health responsibilities and in accordance with the Seasonal Influenza ES.
B5. General
18. What are the reasonable adjustments that may need to be made to enable someone with a learning disability to get their influenza vaccination?
People with a learning disability are entitled to receive an influenza vaccination under the terms of the Seasonal Influenza ES and the Pharmacy first advanced service.
People with a learning disability should be invited for an influenza vaccination if they are on a practice’s learning disability register, so it is important practices ensure their registers are up to date.
There is a legal duty to make ‘reasonable adjustments’ for people with a learning disability. This means making changes to services so that they are easier for people with a learning disability to use for example: considering the accessibility of the environment (reception, waiting area, consulting room), adjusting timing of appointments and length of visit.
See resources on annual health checks and influenza vaccinations for people with a learning disability including easy read information.
Practices should be mindful of the accessibility needs for all their patients and make reasonable adjustments as required.
19. What system must be used to record influenza vaccinations administered by a PCN grouping as part of a COVID-19 Collaboration Agreement and how will the practices receive payment?
The host practice nominated to receive the payment for COVID-19 vaccinations may elect whether to use either GP IT system (GPES and CQRS) or the Point of Care System for recording vaccination events. If the PCN groupings elect to record in the Point of Care System they must only record each vaccination event in the Point of Care System and not their GP IT clinical systems.
IoS payments for the administered influenza vaccinations (£10.06) will be made to the PCN grouping’s nominated host practice. Practices must, within their PCN groupings, determine how these payments are then shared between the collaborating practices.
20. What system must be used to record influenza vaccinations administered by practices working together as part of an Influenza Collaboration Agreement and how will the practices receive payment?
Practices collaborating to deliver influenza vaccinations must only record each vaccination event in GP IT clinical systems and not a Point of Care system. IoS payments for the administered influenza vaccinations (£10.06) will be made accordingly to each practice. Practices must, within their collaboration and where applicable determine how these payments are then shared between the collaborating practices.
Publication reference: PRN01461_ii

