Equality and health inequalities
1. Promoting equality and addressing health inequalities are at the heart of NHS England’s values.
2. Through research and innovation we can make an important contribution to reducing health inequalities by ensuring equitable and widespread access to proven innovations for all, and the adoption of innovations that proactively address and reduce inequalities in healthcare experience or outcomes.
3. The MedTech Funding Mandate (MTFM) policy works to address health inequalities in 3 ways:
- striving for equitable access by ensuring all eligible patients can access the latest proven cost-saving National Institute for Health and Care Excellence (NICE) technologies; access to their use across England is mandated through the MTFM funding mechanism
- ensuring our approach facilitates access by groups disproportionately affected by health inequalities as identified by the Core20PLUS5 approach
- including technologies that treat conditions that disproportionately affect certain patient groups, such as Spectra Optia for patients with sickle cell disease
4. Local commissioners and providers have a central role in using innovation to reduce inequity in patient access, experience, and outcomes through the implementation of the MTFM policy.
5. The Innovation, Research, Life Sciences Strategy (IRLSS) Patient and Public Involvement team empower patient groups and charities by communicating information on the new treatments supported by the MTFM policy. This helps patients understand what the best treatments are that should be available to them from their healthcare provider and supports them to have informed discussions with their clinicians.
Introduction
6. In the NHS Long Term Plan, NHS England outlined how research and innovation would drive better outcomes and experience for patients. An important element was the commitment to introduce a MedTech Funding Mandate (MTFM) policy to accelerate the adoption of selected NICE recommended, cost-saving medical devices, diagnostics and digital products in the NHS, meaning patients will get access to these technologies across England by removing funding barriers. The MTFM policy was launched in April 2021 with this document providing a policy update, effective from 1 April 2024.
7. This update introduces a new technology to be supported by the MTFM policy. New information is also included on the type and duration of support given to technologies by both the Health Innovation Network and NHS England. New data dashboards have been developed and details of how to access them have been included.
8. The Life Sciences Vision, published in July 2021, set out the government’s ambitions to build on scientific successes and tackle future challenges. It recognised the opportunity to accelerate the development of MedTech tools and get them to patients more quickly and refers to the MTFM policyas playing a critical role in addressing the most pressing needs of the NHS in England.
9. The MTFM policy builds on prior NHS Accelerated Access Collaborative (AAC) innovation programmes, such as the Innovation and Technology Tariff/Payment Programme (ITT/ITP), introduced to address financial and procurement barriers to the adoption of devices, diagnostics and digital products.
10. The aim of the MTFM policy is to accelerate equitable patient access to medical technologies that are clinically effective, cost saving in 3 years (as determined by NICE) and affordable to the NHS (costs not exceeding £20 million), by supporting their implementation and then scaling them (adoption and spread).
11. To achieve the aim, the objectives of the MTFM policy are to:
- mandate commissioners to fund the MTFM technologies when clinically appropriate
- ensure equity in healthcare provision is achieved by monitoring patient access to the supported technologies across the NHS in England
- direct the NHS on which MedTech innovations are effective and likely to give savings on investment
- support the NHS to develop a sustainable approach to overcoming the financial barriers to adopting medical devices, diagnostics and digital products
12. In preparation for FY 2024/25, the MTFM Policy team have worked with NICE and other key partners to assess all available medical technology guidance and diagnostic guidance.
13. We are adding one new technology to the MTFM from 1 April 2024. Systems should continue to prioritise the appropriate adoption of all supported technologies, which offer cost savings and improved patient outcomes and experiences.
14. The IRLSS group, along with NICE, NHS Supply Chain and the Health Innovation Network (formerly the Academic Health Science Network) will continue to support national adoption of the supported technologies.
15. The MTFM policy is for NHS providers and their commissioners in England, and explains:
- the scope of the MTFM policy
- which technologies are included
- the implementation support available via NICE tools and resources, the Health Innovation Network and NHS England
- the roles and responsibilities of NHS providers, NHS commissioners and suppliers of technologies, and how they will be supported by the MTFM policy and the Health Innovation Network
- plans for performance and evaluation, and compliance monitoring
Scope
16. The MTFM policy was launched on 1 April 2021. This updated guidance replaces previous versions and is effective from 1 April 2024.
17. NHS England’s IRLSS team assessed relevant NICE guidance to understand which technologies met the criteria for inclusion in the 2024/25 MTFM policy. We will continue to consider technologies proven to be clinically effective and cost-saving for inclusion in updates of this policy, with support from NHS providers, NHS Integrated Care Systems, NHS Supply Chain, the Department of Health and Social Care, Patient and Public Voices, industry representatives and representative bodies for clinicians.
18. In the NHS Standard Contract, General Service Conditions section 2.2 states that “The Parties must comply, where applicable, with their respective obligations under, and with recommendations contained in, MTFM guidance”.
Criteria for inclusion in the MedTech Funding Mandate
19. In 2023/24, we reviewed published NICE guidance to identify devices, diagnostics or digital products that:
- are effective: demonstrated through positive NICE guidance (1, 2)
- are cost saving within 3 years: NICE modelling demonstrates a net saving in the first 3 years of implementing the technology (3)
- are affordable to the NHS: the cost should not exceed £20 million in any of the first 3 years (4)
Notes:
(1) Please refer to information on NICE guidance.
(2) We reserve the right not to include a technology in the MTFM policy and/or to undertake further negotiations with technology suppliers if additional data collection is required to demonstrate sustained effectiveness.
(3) Demonstrated by a NICE published resource impact assessment (RIA).
(4) We reserve the right not to include a technology in the MTFM policy and/or to undertake further commercial negotiations with manufacturers if we believe the £20 million cost limit will be exceeded in any of the first 3 years.
20. NHS England works closely with NICE to ensure that the MTFM criteria align with future changes to the medical technology evaluation process and subsequent guidance publication process.
21. At the time of updating this policy document, NICE is in the process of transforming its methods and processes for the evaluation of outputs to multi-tech assessments. It is also developing its lifecycle evaluative approach to health technologies, capturing the value of innovation at the different stages of technology development. Technologies will be grouped into three broad categories.
- Early Value Assessment (EVA) for new technologies with emerging evidence;
- Medical Technology Guidance (MTG) for new technologies ready for widespread NHS adoption;
- Late-Stage Assessment (LSA) for technologies within a category, currently chosen by DHSC, whose use is already in the NHS.
22. The outcome of the NICE transformation programme could have implications for the policy criteria and technology selection process. NHS England will continue to work with NICE to understand how the MTFM policy could be adapted to accommodate any future changes.
23. Any changes to the MTFM policy, including criteria and processes, will be signalled on the MTFM webpage.
How technology on the MTFM is supported
24. We have developed a strategy for ensuring that industry and the health sector understand how long a technology will be supported by the policy, and what the package of support includes.
25. We aim to support the national spread and adoption of each technology for 3 years. This support will be tailored to the technology, considering any barriers and enablers to adoption, and will be agreed by the Health Innovation Network National Programme Director, Health Innovation Network Technology Lead, the MTFM policy team and the technology supplier. The factors to be considered in planning a programme of support include the degree of clinical consensus, workforce impacts, the required patient pathway transformation and the maturity of a technology.
26. We will provide support in 3 phases as follows:
- Phase 1: 6 months of ‘onboarding’ support. This is before the new technology is supported by the MTFM. The Health Innovation Network will work with NICE and the MTFM Policy team to understand the scale of pathway transformation a technology needs to deliver its benefits.
- Phase 2: 2 years of ‘active support’. This is when technologies are supported by the MTFM policy. The Health Innovation Network will raise awareness of the technology, support implementation and drive its adoption throughout the country.
- Phase 3: 6 months of ‘graduation to business as usual’ support. This supports the transition to business as usual, and during this phase organisations will continue to have access to Health Innovation Network guidance and tools (such as implementation plans). Beyond this phase, the payment mechanisms and requirement for integrated care boards (ICBs) to support new implementations will still apply, and the Health Innovation Network will continue to support active business cases until a technology is fully implemented.
27. The level of support for each technology will depend on progress with adoption. Annex 4 breaks down the typical level of support each technology will receive.
28. The MTFM Policy team and the Health Innovation Network will deviate from the above programme of support under the following exceptional circumstances:
- the NICE guidance for the supported technology changes significantly. For example, if new evidence emerges around the efficacy of a technology and NICE no longer recommends its use in the NHS
- new innovations remove or significantly reduce the need for the NHS to adopt a current MTFM product
- the system faces significant barriers to the adoption of a MTFM product. This could be due to clinicians raising concerns about a particular technology, where emerging findings misalign with the NICE guidance. In these cases, we would work closely with NICE to share feedback and agree an approach
- adoption levels for a technology are static for a sustained period (~6 months) and further progress is unlikely. Adoption can be monitored by triangulating NHS Supply Chain data, supplier insights and Health Innovation Network Quarterly Assurance Reporting Tool (QART) data. In this circumstance, a review meeting with all parties would be needed to agree an approach
- a supported technology is no longer available in the UK market due to supplier withdrawal, significant supply chain issues or other commercial changes
- a change in the price of the MTFM product or an alternative treatment invalidates the resource impact assessment and means the supported product is no longer cost saving
- a supplier asks to withdraw its product from MTFM support
- a supplier fails to adhere to the code of ethical business practice as set out by the Association of British HealthTech Industries
- a supplier fails to adhere to the MTFM data sharing requirements outlined in the memorandum of understanding agreed in the onboarding phase
29. Technology support issues will be raised through the policy compliance process and actions agreed by the policy governance process by the HIN.
2024/25 MedTech Funding Mandate technologies
30. The technologies selected for support in 2021/22 have now graduated to ‘business as usual’. As explained in paragraph 26, the policy levers remain in place for those still wishing to implement these technologies from 2021/22:
- placental growth factor-based testing (PLGF) (DG49) (1) – a diagnostic test to help rule out pre-eclampsia (Triage PlGF test and the Elecsys immunoassay sFlt-1/PlGF ratio)
- SecurAcath (MTG34) – for securing percutaneous catheters
- HeartFlow FFRCT (MTG32) – for estimating fractional flow reserve from coronary CT angiography
- gammaCore (MTG46) – a handheld device that alleviates the symptoms of severe cluster headaches by stimulating the vagus nerve
Notes:
(1) DG49 replaced DG23, which has been supported by the MTFM policy since 2021. DG49 was published on 27 July 2022. Currently the MTFM policy only supports 2 of the 3 technologies recommended in DG49.
31. Since 2022/23, 7 more technologies have been supported. These will be supported with a further 6 months of phase 2 support and will then be reviewed and begin their phase 3 support from September 2024.
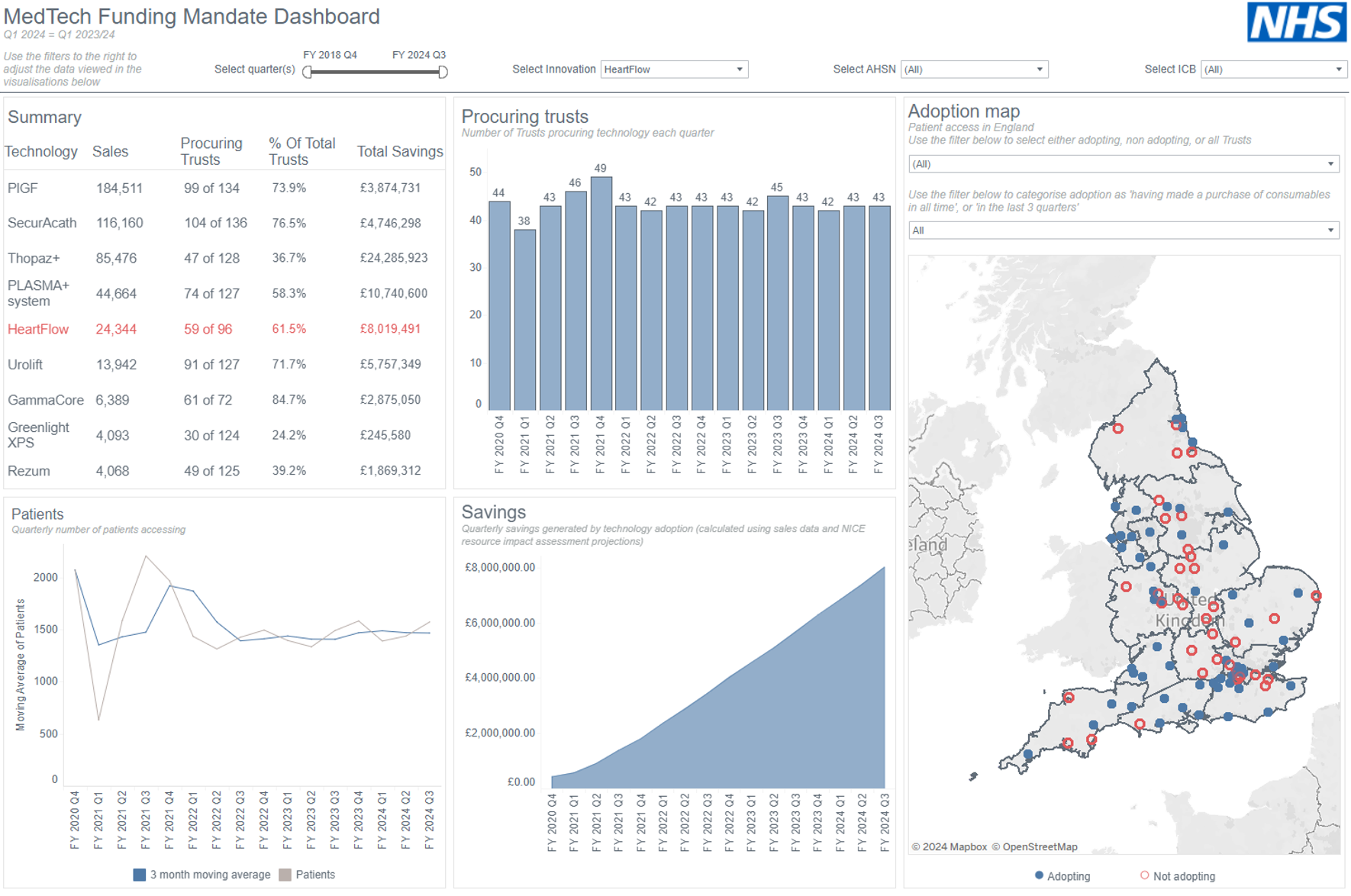
32. 4 of these technologies are an alternative treatment to transurethral resection of the prostate (TURP) for benign prostatic hyperplasia.
- GreenLight (MTG74) – uses a laser to reduce the size of an enlarged prostate
- Rezum (MTG49) – uses water vapour to destroy excess prostate tissue
- PLASMA System (MTG53) – uses electrodes to cut out prostate tissue
- UroLift (MTG58) – lifts and holds the enlarged prostate tissue away from the urethra, relieving the compression of this organ
33. The remaining 3 technologies supported from 2022/23 are an alternative to more invasive procedures:
- Spectra Optia (MTG28) – apheresis and cell collection platform for people with sickle cell disease who require automated red cell exchange
- XprESS Multi Sinus Dilation System (MTG30) – a sterile, single-use device for treating chronic sinusitis with a dilating balloon
- Thopaz+ (MTG37) – a portable digital chest drain system that accurately monitors and records air leak and fluid drainage.
34. For 2024/25, the policy will support one more technology:
- AposHealth (MTG76) – a medical device that patients with knee osteoarthritis wear on a foot to redistribute their weight and adjust their gait to reduce long-term join pain.
35. Further detail on these technologies can be found in Annex 1.
36. Newly published NICE guidance is assessed to understand if the technology meets the criteria in paragraph 18. The MTFM team also carry out assessments to ensure the technology:
- aligns to NHS England’s priorities
- is supported by NHS clinical leads
- aligns to Get It Right First Time programmes
- is suitable for use in the acute sector
37. Once a technology is understood to be suitable for support under the policy, it will be signalled on the policy webpage to enable the sector to prepare for its inclusion in activity planning.
38. With NICE, we will continue to monitor the spread, adoption and real-world evidence from the implementation of these technologies, and compare this with published NICE guidance and tools.
39. Should a technology no longer meet the criteria for policy support, this finding will be communicated on the policy webpage.
40. For all technologies supported under the MTFM policy, please refer to the NICE guidance to understand implementation eligibility.
41. Technologies are funded by local commissioners from existing allocations, except for Spectra Optia.
42. Spectra Optia is for the treatment of sickle cell disease, which is part of the Blood and Infection National Programme of Care (NPoC) that provides leadership and oversight of haemoglobinopathies. NHS England commissions these services. Therefore, Spectra Optia costs will be funded from existing NHS England allocations.
43. A Spectra Optia working group has been created to ensure that patients with sickle cell disease have access to automated red cell exchange procedures, particularly out of hours. The group are working with the 10 haemoglobinopathy co-ordination centres and the National Blood Transfusion Service to understand where additional capacity and out-of-hours access to the equipment are needed, and how this can be supported by NHS England Specialised Services commissioning.
44. Providers that want to know more about this should contact the MTFM inbox medtechfundingmandate@nhs.net in the first instance.
Communication of future changes to MTFM policy criteria and products
45. The technologies already covered by the MTFM policy will be subject to review to determine if any should be removed, including those for which NICE guidance has been significantly updated; alternative treatment or diagnostic options exist; or significant safety concerns have been raised. If any technologies are to be withdrawn, this will be signalled ahead of further policy publication, via the policy webpage.
46. Removal of technologies will be signalled in year via the policy webpage, giving commissioners and providers that may be impacted by the change time to prepare.
47. The technologies covered by the MTFM policy will be updated annually in related NHS England publications, including the NHS Payment Scheme and NHS Standard Contract. These are typically published on the NHS England website between December and March and are subject to their own consultation processes.
48. The MTFM policy will be updated in line with any relevant and significant legislative changes.
49. We invite stakeholders to join our FutureNHS page to get the latest updates on the MTFM policy, including information on engagement events, the technologies supported and planned publications, including the signalling of the next year’s technologies. Please log onto FutureNHS and search for the MTFM workspace to request membership.
NHS Payment Scheme
Financial impact
50. The MTFM policy does not provide additional funding for the technologies it supports. Instead, it mandates commissioners to fund the MTFM technologies when clinically appropriate. The MTFM policy criteria ensure that technologies are cost saving within 3 years, as estimated by the NICE Resource Impact Assessment (RIA) team.
51. The savings analysis from NICE can be found in Annex 2.
52. NICE also produces tools to help providers and commissioners understand the technology’s impact on their patient population; see Annex 1.
NHS Payment Scheme 2023/25
53. The 2023/25 NHS Payment Scheme (NHSPS) excludes the cost of MTFM technologies from core payment mechanisms (see NHSPS, Section 3.4, Excluded items).
54. The list of MTFM items is included in Annex A of the NHSPS.
55. More details are also available in Appendix 3 of the supporting document.
56. A guide to applying the NHSPS to possible MTFM policy scenarios can be found in Annex 3.
Funding the cost of the technologies
57. The MTFM policy has a ‘pass through’ payment approach, where the commissioner is required to pay for the cost of MTFM technologies from existing allocations on a ‘cost and volume’ basis, where clinically appropriate. The MTFM technologies are excluded from core payment mechanisms and a list of supported technologies is published on tab 12c ‘MedTech FM products’, NHSPS Annex A.
58. Items on the ’12c MedTech FM products’ list are subject to the NHSPS excluded items pricing rule (Section 3.4). This stipulates that the price the commissioner pays must reflect actual costs, the prices set under any applicable procurement framework or a reference price set by NHS England, whichever is the lowest.
59. NHS England has not set any reference prices for the technologies supported. Therefore, actual cost should be reimbursed by commissioners to providers.
Funding the capital purchase
60. Technologies supported by the policy that are capital purchases; for example, Spectra Optia apheresis machine, should be added by providers to their priority capital spend list. On the completion and approval of a business case, the depreciation should be reimbursed by the
Funding the cost of implementation
61. Funding of the technologies does not include implementation or running costs: this was highlighted as a barrier to adoption. Therefore, the guidance set out in Appendix 3 of NHS provider payment mechanisms states that the API fixed payment agreed between commissioners and providers should include all known upfront implementation costs.
62. The Health Innovation Network has been commissioned to support local systems with implementation plans and can help providers define local implementation resourcing and costs of the different technologies.
Tools to support commissioner agreements
63. The NHS England innovation payment project developed guidance to help commissioners and providers navigate through the NHSPS for 2023/25. Providers and commissioners need to understand the cost of both implementation and any potential running costs of introducing a new technology to a specialty, to include these costs in the fixed element of their agreements.
64. Annex 3 explains how the payment mechanisms can be applied to different MTFM implementation scenarios.
65. NICE provides tools and resources as part of the published guidance to help with this. NHS England, the Health Innovation Network and technology suppliers will build business cases and case studies to help understand the technologies and their benefits; these are available on the FutureNHS page.
National Cost Collection 2024
66. The 2024 National Cost Collection will include cost and activity data for the technologies supported in the policy.
67. The 4 technologies supported in 2021/22 were all centrally funded by the ITP Programme, where payments were made directly to the supplier at zero cost to providers. From April 2021, it was the provider’s responsibility to pay for the technologies themselves, meaning the cost of the technologies is visible and can be included in local service line reporting (SLR) information and in the National Cost Collection data. (Note: SLR is not mandated and the frequency of reporting is locally determined. Please contact your provider’s costing team for more information.)
68. As part of an update to the costing standards, NHS England has added functionality to the integrated technical document to enable providers to identify the technologies supported by the MTFM policy in their cost data.
69. A new collection resource code was added to the National Cost Collection from 2022, and NHS provider costing practitioners should use this to identify on submission the cost of the technologies supported by the MTFM policy. The collection resource code will enable NHS England to analyse the use of the technologies nationally and facilitate benchmarking and opportunity analysis within The Model Health System, a data-driven improvement tool enabling quality and productivity benchmarking..
70. National cost data will also enable local and national analysis to demonstrate the cash-releasing and resource-saving benefits from adopting the technologies in the policy.
Procurement of MTFM technologies
71. Technologies included in the MTFM policy should be procured through the relevant NHS Supply Chain framework, giving providers a procurement route that means they do not have to negotiate individually with suppliers.
72. NHS Supply Chain will support marketing of the policy through customer communications, NHS Supply Chain webpages, and supporting national webinars.
73. NHS Supply Chain Care Pathway team will support system implementation in collaboration with the health innovation networks.
74. NHS England will continue to work with NHS Supply Chain to understand the feasibility of moving to a more robust reporting mechanism. Updated guidance will be issued and any developments will be communicated on the policy webpage and the FutureNHS page.
75. Providers of NHS-funded services can set up an NHS Supply Chain account via the online catalogue and ordering accounts webpage.
76. Non-NHS providers of NHS-funded services can apply for an NHS Supply Chain account via the create an account webpage.
77. NHS Supply Chain’s guidance notes to its online catalogue and ordering provides step-by-step guidance for ordering via the NHS Supply Chain online catalogue.
Performance and evaluation
Spread and adoption of MTFM technologies
78. NHS England will review the stage of adoption of MTFM technologies, by provider site, using the QART. This informs the reporting for the MTFM data dashboard, which is published on FutureNHS page (access will need to be requested) and used to demonstrate the extent of adoption for each technology, the numbers of patients accessing it, as well as its estimated impact. An example of the dashboard’s output is shown below:
79. Health innovation networks also report on the stage of adoption each eligible provider is at for respective MTFM technologies. This information is reported in a more detailed operational dashboard only accessible to Health Innovation Network and NHS staff. To ensure consistency, the QART stage of adoption reported for each technology must adhere to the following definitions:
| Stage | Definition |
|---|---|
| 0: No information | No information is known, or contact is not yet established with the Provider |
| 1: Knowledge | Provider has knowledge of the NICE guidance |
| 2: Interest | Efforts have been made by the Provider to gain information about the NICE guidance |
| 3: Decision | Provider and/or commissioner has made a firm decision not to implement the NICE guidance (and why) |
| 4: Implementation | Provider has agreed with commissioner to implement the NICE guidance |
| 5: Adoption | Patients are benefitting from the NICE guidance at the Provider |
80. The reporting identifies which providers and commissioners have yet to adopt a technology, supporting discussion on their current barriers and helping them learn from other providers and commissioners that have successfully adopted the technology.
81. NHS England has worked to strengthen the data reporting using sales data reported by the supported technology suppliers. Work to develop intelligence on the impact of the MTFM technologies is ongoing. Updates on this work will be published on the policy webpage and FutureNHS page.
Monitoring compliance
82. The 2024/25 NHS Standard Contract will “require both commissioners and providers of NHS-funded services to comply, as relevant, with their obligations under, and any recommendations contained in, the MedTech Funding Mandate”. This builds on the existing contractual requirement to have regard for guidance published by NICE. To be compliant, we would expect eligible patients to be able to access the technologies supported by the policy.
83. Patients have the right to access drugs and treatments that have been recommended by NICE for use in the NHS, if their doctor says they are clinically appropriate for them in accordance with the NHS Constitution for England.
84. Compliance is not relevant where the NICE recommendations are not relevant to the organisation; for example, the provider does not provide services for the specific patient cohort the technology supports, or an alternative treatment is more appropriate for a patient.
85. Technologies included in the MTFM policy have been proven to support safe and effective care and the Care Quality Commission (CQC) can use evidence of their adoption as evidence that a provider is meeting its regulatory requirements.
86. Together with the strengthened NHS Standard Contract requirement to comply with the MTFM, and patient awareness that these technologies must be a treatment option in line with NICE recommendations, providers may wish to review how they demonstrate their compliance with the MTFM.
87. Examples of how NHS commissioners and providers of NHS-funded services can evidence compliance with MTFM policy guidance include:
- Integrated care system (ICS) publishing policy statements, service-level agreements and/or contracts to demonstrate funding is in place and that they require innovations covered by the MTFM policy to be available for use, in consultation with the patient and when recommended by NICE as part of their treatment pathway.
- Providers of NHS-funded services publishing their policies and clinical care pathways to demonstrate that innovations covered by the MTFM policy are available and evidenced as part of the safe, effective and/or well-led sections of the CQC assessment framework.
- Organisations publishing audit data and patient surveys to demonstrate the use of technologies covered by the MTFM policy
88. We will continue to work with the Health Innovation Network and NHS Supply Chain to track the adoption of the technologies covered by the MTFM policy. Uptake data will be included in the MTFM dashboard and monitored through the AAC Board. When NHS England is made aware of non-compliance, we will seek to engage with the relevant providers and commissioners to provide support, and understand the barriers they are facing and how these can be overcome. This is to ensure the MTFM policy aim of equitable health access across England is achieved.
Implementation support
Health Innovation Network
89. Providers of NHS-funded services and NHS ICSs have access to implementation support from the 15 health innovation networks across England.
90. NHS England established these networks in 2013 to spread innovation, improve health and generate economic growth. Each Health Innovation Network works across a distinct geography and is connected to the regional and local NHS structures.
91. The health innovation networks connect NHS and academic organisations, local authorities, charities and industry, and provide a range of practical support to facilitate change across health and social care economies.
92. The Health Innovation Network have extensive experience of implementing these technologies in the NHS, having supported the national adoption and spread component of the ITT/ITP programmes.
93. The Health Innovation Network can bring together the appropriate people in clinical services, finance, procurement, and commissioning within an ICS link provider clinical teams to the corporate teams and commissioners, assist with planning discussions and support business case development for initial and/or sustained adoption.
94. In addition, the Health Innovation Network is working with the NHS Supply Chain Care Pathway team to bring their experience and knowledge of the procurement and financial landscape to the implementation of the MTFM policy.
95. To contact your local Health Innovation Network for support, please visit its website.
NICE tools and resources
96. NICE develops tools to help providers of NHS-funded services implement NICE guidance. These include:
- costing statements/resource impact reports explaining the resource impact guidance
- resource impact templates to help local areas assess the financial impact of the guidance
- general implementation materials outlining how to put guidelines into practice
- specific examples developed with providers that have implemented the technologies, which include:
- plain language ‘information for the public’ summaries of the technologies
- shared learning case studies from NHS organisations that have implemented the technologies
- checklists
- data protection agreements
97. Links to the NICE implementation support materials for these technologies are provided in Annex 1.
Roles and responsibilities
98. This section describes the roles and key responsibilities for MTFM policy stakeholders including:
- suppliers of technologies included in the policy
- NHS England
- Health Innovation Network technology leads
- individual Health Innovation Networks
- NHS providers – corporate and clinical teams
- NHS ICSs
Suppliers of technologies supported by the MedTech Funding Mandate
99. Technology suppliers are expected to:
- consider additional resource and capacity for the scaling up of their business to meet increased demand if this is relevant
- produce high quality business cases for commissioner funding if providers require funding to purchase the technology
- work with NHS Supply Chain in readiness for the effective date and onboard their respective providers
- support the Health Innovation Network with relevant communications and engagement including action learning sets; and support clinicians in relevant discussions involving the technology
- adhere to the code of conduct as set out by the Association of British HealthTech Industries (ABHI) or MedTech Europe, and respect that being included in the MTFM policy is not a sales opportunity; the focus is on equitable patient access
- work with the MTFM Policy team and IRLSS Performance and Evaluation team to monitor patient access by sharing sales data
NHS England
100. NHS England is expected to:
- engage with NICE, the Health Innovation Network, Supply Chain Coordination Ltd (SCCL; the management function for the NHS Supply Chain operating model) and NHS Supply Chain to develop and improve MTFM policy documents and operational processes
- produce MTFM policy documents, tools and engagement pieces, including patient and public involvement
- prepare Health Innovation Network leads and suppliers for the policy effective date by sharing knowledge on NHS processes including, but not limited to, procurement and funding mechanisms
- support non-compliance resolution to ensure the aims of the MTFM policy are being met
- support resolution of issues should any concerns be raised in relation to the technology suppliers’ business conduct
Health Innovation Network technology leads
101. Health Innovation Network technology leads are expected to:
- lead Health Innovation Network baselining activity for technologies to understand current adoption across England and work with NICE on findings
- develop a suite of implementation support tools for all Health Innovation Network and system stakeholders to use in product adoption and spread, including an implementation toolkit and business case template, by working with NICE and the product supplier
- be the focal point for gathering learning from across the Health Innovation Network on barriers and success stories
- help overcome clinical barriers to adoption by offering advice and guidance to all 15 health innovation networks, working with clinical champions where appropriate
- work with NHS England to develop case studies and deliver engagement pieces, including webinars, to support implementation and spread
- report national progress to the Health Innovation Network National Programme Director to support governance processes
Individual health innovation networks
102. Individual Health Innovation Networks are expected to:
- support business case production by sharing templates and examples, and with quality assurance
- understand adoption status across all eligible provider sites
- raise awareness of MTFM policy and products across the local system
- be honest brokers between product suppliers and NHS teams
- share best practice in implementation from other NHS systems
- support collection of evidence to demonstrate impact of the product
- when alternative technologies to those supported by the MTFM policy are in place, support the collation of evidence to demonstrate equivalent outcomes for discussion with the IRLSS team and NICE
- capture and report barriers and issues across the 15 health innovation networks share with the IRLSS team and NICE
- escalate non-compliance to the IRLSS team by sharing all available knowledge and understanding
NHS Supply Chain
103. NHS Supply Chain are expected to:
- provide procurement knowledge and insight of the market and supply chains
- procure in readiness for the effective date and onboard respective suppliers and providers
- collaborate with the health innovation networks to implement the MTFM policy, using the experience and knowledge of the NHS Supply Chain Care Pathway team
- work with NHS England to monitor patient access by sharing sales data
- raise awareness of the MTFM policy and supported products across the local system
- collaborate with NHS England to develop improved processes and tools
NHS providers – corporate teams
104. Provider corporate teams are expected to:
- familiarise themselves with the MTFM policy guidance
- work with the Health Innovation Network and local teams to understand which technologies their health system is eligible to provide (NICE guidance, tools and resources will assist this)
- engage with the NHS Payment Scheme guidance, tools and communications that support the MTFM policy
- work with commissioners to understand the initial funding requirements to implement technologies, future financial benefits and the value of future capacity benefits
- collaborate with the Health Innovation Network and clinical teams to understand the technologies and their respective benefits to services
- engage commissioning and costing teams to plan current and future contracting arrangements that include the MTFM technologies
- join the MTFM FutureNHS workspace to be aware of any planned engagement events and policy developments
NHS providers – clinical teams
105. Provider clinical teams are expected to:
- collaborate with health innovation networks and suppliers to understand the technologies and their respective benefits to services
- engage with their corporate teams to help them understand which technologies the provider is eligible to provide in line with NICE guidance (see Annex 1)
- raise patient awareness of new available treatments and their benefits
- work with specialty clinicians to prepare for changes to care pathways
- agree the expected level of activity for each technology
- promote service improvements with primary care services
- record data on outcomes and benefits achieved through technologies and share this appropriately
- join the MTFM FutureNHS workspace, which provides updates on planned engagement events and policy developments
NHS ICBs
106. NHS ICBs are expected to:
- familiarise themselves with the MTFM policy guidance, paying particular attention to Annex 3
- engage with the NHS Payment Scheme guidance, tools and communications that support the MTFM policy
- identify local patient populations that technologies will benefit (using NICE resource impact templates)
- engage with providers to agree projected activity and how this fits in with contractual arrangements
- work with providers and ensure funding is made available
- monitor evidence of spread and adoption, and benefits to patients
- join the MTFM FutureNHS page, which provides updates on planned engagement events and policy developments
Annex 1: Innovations supported by the MedTech Funding Mandate
HeartFlow (MTG32)
HeartFlow FFRCT estimates fractional flow reserve from coronary CT angiography (CCTA) for patients with stable, recent-onset chest pain.
- NICE medical technologies guidance (MGT32): HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography
- HeartFlow® product website
Clinical benefit (as stated by NICE)
HeartFlow FFRCT is as accurate as CCTA in excluding coronary artery disease and characterises the coronary arteries from both functional and anatomical perspectives, differentiating between ischaemic and non-ischaemic vessels in a way that CCTA cannot. The coronary lesions responsible for coronary artery disease can be identified without the need for invasive procedures and further non-invasive tests.
Patient benefit (as stated by NICE)
- replaces the need for an invasive procedure in a specialist cardiology procedure suite
- reduced length of stay
- reduced hospital visits as multiple diagnostic tests such as exercise tests and stress tests are not required
- faster diagnosis
- reduced waiting times for patients waiting for a procedure in the specialist cardiology procedure suite
SecurAcath (MTG34)
SecurAcath is a device to secure peripherally inserted central catheters (PICCs) and should be considered for any PICC with an anticipated medium to long-term dwell time (15 days or more).
- NICE medical technologies guidance (MTG34): SecurAcath for securing percutaneous catheters
- SecurAcath product website
Clinical benefit (as stated by NICE)
SecurAcath is easy to insert, well tolerated, associated with a low incidence of catheter-related complications and does not usually need to be removed while the catheter is in place. Clinical benefits include no interruptions or delays from the catheter becoming dislodged. SecurAcath improves vessel preservation and reduces need for re-insertions. There are also fewer complications such as migration, thrombosis and infection.
Patient benefit (as stated by NICE)
- no risk of medical adhesive-related skin injury
- no requirement for frequent adhesive fixing changes
- reduced risk of interruption to treatment
- reduced risk of catheter-related infection
- reduced pain on insertion and while in situ
gammaCore (MTG46)
gammaCore (electroCore) is a non-invasive vagus nerve stimulator used to treat and prevent cluster headaches. It is self-administered by the person or their carer.
Clinical benefit (as stated by NICE)
Clinical evidence shows that, for some people, using gammaCore as well as standard care reduces the frequency and intensity of cluster headache attacks and the need for medication. This is likely to significantly improve quality of life for people living with this condition.
Patient benefit (as stated by NICE)
- significant quality of life improvement from reduced pain during an attack
- reduced need for expensive medication
- reduced hospital visits
PLGF (DG49)
Placental growth factor (PLGF)-based tests are intended to be used with clinical judgement and other diagnostic tests, to help rule out suspected pre-eclampsia. This assessment focuses on ruling out pre-eclampsia in the second and third trimesters of pregnancy.
- NICE diagnostic guidance DG49: PLGF-based testing to help diagnose suspected pre-eclampsia
- Roche Elecsys sFlt-1 PLGF product website
- Quidel Triage PLGF product website
Clinical benefit (as stated by NICE)
Using PLGF-based tests in addition to standard clinical assessment promotes better risk assessment for adverse outcomes in women with suspected pre-eclampsia. It allows people in whom pre-eclampsia has been ruled out with a PLGF-based test to return to community care instead of being admitted to hospital for observation.
Patient benefit (as stated by NICE)
- reduced length of stay if patient already admitted
- admission avoidance if test carried out without admission to hospital
- reduced need for further third trimester scans
- increased assurance reduces stress for patients
UroLift (MTG58)
The UroLift system is an implanted device which lifts and holds enlarged prostate tissue away from the urethra, relieving the compression of this organ. It can be implanted under local anaesthesia in an outpatient setting or ambulatory care centre, and the patient can return home the same day without a catheter.
- NICE Medical technologies guidance (MTG58): UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia
- UroLift® product website
Clinical benefit (as stated by NICE)
UroLift relieves lower urinary tract symptoms for up to 5 years. It also improves quality of life and avoids risk to sexual function.
Patient benefit (as stated by NICE)
- it is for people aged 50 and older with a prostate of 30 to 80 mL
- the procedure is minimally invasive, so open surgery is not needed, and it does not affect sexual function
- it can usually be implanted without an overnight stay in hospital, allowing the patient to return home without a urinary catheter
GreenLight XPS (MTG29)
GreenLight XPS vaporises prostatic tissue with a laser. The laser fibre is passed through a cystoscope to photoselectively vaporise the enlarged prostate tissue, leaving a clear urethral channel. GreenLight XPS can be done as a day-case procedure, reduces the risk of complications and allows a quicker return to normal activity.
- NICE Medical technologies guidance (MTG29) GreenLight XPS for treating benign prostatic hyperplasia
- Boston Scientific GreenLightTM product website
Clinical benefit (as stated by NICE)
GreenLight XPS uses a laser to reduce the size of an enlarged prostate, easing the symptoms of benign prostatic hyperplasia (BPH).
Patient benefit (as stated by NICE)
- can more often be done as a day-case procedure (patients can go home on the same day as the procedure is done)
- it allows patients to get back to normal day-to-day activities quicker after the procedure
Rezum (MTG49)
Rezum is a minimally invasive procedure that uses water vapour (steam) to treat BPH. The technology delivers targeted, controlled doses of stored thermal energy in water vapour directly to the region of the prostate gland with the obstructive tissue causing lower urinary tract symptoms (LUTS).
Rezum effectively alleviates BPH and patients can be treated as outpatients.
- NICE Medical technologies guidance (MTG49): Rezum for treating lower urinary tract symptoms secondary to benign prostatic hyperplasia
- Boston Scientific RezumTM product website
Clinical benefit (as stated by NICE)
Rezum uses water vapour to destroy excess prostate tissue with the aim of relieving symptoms. The process is intended to disrupt cell membranes, leading to cell death and shrinking the prostate. The intention is to relieve obstructive symptoms without interfering with surrounding tissues that might impair sexual function.
Patient benefit (as stated by NICE)
- relieves symptoms
- improves quality of life
- minimally invasive, which means open surgery is not needed
- unlikely to need a stay overnight in hospital
PLASMA System (MTG53)
PLASMA is a bipolar electrosurgery system for TURP. The system uses electrodes to cut out (resect) prostate tissue and stop any local bleeding afterwards (haemostasis), which avoids the risk of transurethral resection syndrome and reduces.
- NICE Medical technologies guidance (MTG53): The PLASMA system for transurethral resection and haemostasis of the prostate
- Olympus PLASMA+ System product website
Clinical benefit (as stated by NICE)
PLASMA avoids the risk of transurethral resection syndrome and reduces the need for blood transfusion. Clinical outcomes are as good as for conventional monopolar TURP but there is a lesser chance of serious complications.
Patient benefit (as stated by NICE)
- less chance of serious complications
- reduces the length of hospital stay
- this procedure can be done as a day case
XprESS multi-sinus dilation system (MTG30)
The XprESS multi-sinus dilation system is a sterile, single-use device for treating chronic sinusitis. Dilation of the XprESS balloon remodels the bony sinus outflow tract by displacing adjacent bone and paranasal sinus structures. This has the potential to reduce the tissue lost compared to traditional functional endoscopic sinus surgery (FESS) procedures.
- NICE Medical technologies guidance (MTG30): XprESS multi sinus dilatation system for treating chronic sinusitis
- Stryker XprESS product website
Clinical benefit (as stated by NICE)
XprESS is a clinically non-inferior, but less invasive, alternative to FESS in patients with uncomplicated chronic sinusitis. Compared with FESS, it may lead to faster recovery times and carries a lower risk of some complications. It has the potential to treat uncomplicated chronic sinusitis earlier in disease progression than is currently available in the NHS. As such, it may improve quality of life and clinical outcomes, as well as reduce surgical waiting lists.
Patient benefit (as stated by NICE)
- an option for chronic sinusitis that has worsened despite drug treatment
- may be beneficial for uncomplicated chronic sinusitis, because it can be done more often under local anaesthesia
- may allow the patient to recover faster than after surgery
Thopaz+ portable digital system (MTG37)
Thopaz+ is a portable digital chest drain system that provides regulated negative pressure close to the patient’s chest and continuously monitors and records air leak and fluid drainage. The system comprises an inbuilt, regulated suction pump with a digital display, rechargeable battery, tubing that connects to any standard chest drain catheter and a Thopaz+ disposable fluid collection canister.
- NICE Medical technologies guidance (MTG37): Thopaz+ portable digital system for managing chest drains
- Medela Thopaz+ product website
Clinical benefit (as stated by NICE)
Sensors in the system turn the pump on and off to ensure the pressure level set by the healthcare professional is precisely maintained. Provides objective measurements of air leakage and fluid loss. This data makes it easier to assess and record patients’ progress. This in turn may help clinicians determine when it is best to remove the chest drain.
Patient benefit (as stated by NICE)
- reduces drainage time
- reduces length of stay in hospital
- allows people to stay mobile during their treatment
- improves safety
- patients may also need fewer chest X-rays with the use of Thopaz+
Spectra Optia (MTG28)
The Spectra Optia Apheresis System is an apheresis and cell collection platform for the treatment of sickle cell disease. In a typical exchange procedure, Spectra Optia separates and removes sickle red blood cells from the patient’s blood using continuous flow and centrifugation. These are replaced with healthy red blood cells according to the user-defined software protocol.
- NICE Medical technologies guidance (MTG28): Spectra Optia for automatic red blood cell exchange in people with sickle cell disease
- Spectra Optia® website
Clinical benefit (as stated by NICE)
Faster and needs to be done less often than manual red blood cell exchange.
Patient benefit (as stated by NICE)
- faster and needs to be done less often than manual red blood cell exchange
AposHealth (MTG76)
AposHealth is a device worn on the foot that improves pain measurement scores, stiffness and function for patients with symptomatic knee osteoarthritis. It is a Class I medical device and is recommended as a cost-saving option to manage knee osteoarthritis in adults where:
- non-surgical standard care has not sufficiently improved the patient’s symptoms
- their condition meets the referral criteria for total knee replacement surgery, but they do not want surgery
- data is collected on the person’s quality of life, health resource use and if they go on to have knee replacement surgery at a later date, after being prescribed AposHealth.
- NICE Medical technologies guidance (MTG76): AposHealth for knee osteoarthritis
Clinical benefit (as stated by NICE)
- Consistent improvement in pain, function and stiffness after using AposHealth.
Patient benefit (as stated by NICE)
- consistent improvement in pain, function and stiffness after using AposHealth
Inclusion criteria
It is important to take into consideration that patients will be individually screened by the treating clinician to confirm suitability for commencing treatment. Referral to surgery is a clinical decision based on a variety of measures.
- Patient diagnosed with knee osteoarthritis where non-surgical standard care has not worked well enough
- Patient’s condition meets the local referral criteria for knee replacement surgery but they do not want surgery
- NICE guidelines on Joint Replacement referral criteria
- Patient’s joint symptoms (such as pain, stiffness, reduced function or progressive joint deformity) are substantially impacting their quality of life
- Non-surgical management (for example, therapeutic exercise, weight loss, pain relief) is ineffective or unsuitable
- Use clinical assessment when deciding to refer someone for joint replacement, instead of systems that numerically score severity of disease.
Exclusion criteria
- severe balance or vertigo issues
- patient relies on a cane or walker to mobilise both indoors and outdoors
- uncontrolled inflammatory condition affecting the knee (for example, rheumatoid arthritis)
- patient has severe neurological, psychiatric or comprehension issues preventing an understanding of how to use the device
- severe vascular occlusions with significant sensory loss
- severe osteoporosis
Example patient profile
A patient with the following characteristics could be considered for treatment with AposHealth:
- knee osteoarthritis (uni/bi/tri compartmental) substantially impacting quality of life and has not responded to standard care
- 40–80 years old (but assessed on functional ability)
- can walk for 5 minutes without support (walking aid)
- requires an alternative to brace or injections (particularly if likely to be non-compliant with these or both likely to be ineffective)
- keen to delay or avoid surgery if possible
- committed to the treatment mode and understands that AposHealth has the potential to help
Annex 2: Estimated resource impact over 5 years according to NICE resource impact assessments
| Product | Estimated cost of current practice £ | Estimated cost of future practice (Y5) £ | Resource impact (Y5) £ |
|---|---|---|---|
| HeartFlow |
67,155,804 |
64,677,299 |
2,478,504 |
| SecurAcath |
5,345,157 |
2,799,535 |
2,545,622 |
| PLGF |
22,193,000 |
17,926,527 |
4,266,473 |
| gammaCore |
216,891,554 |
214,089,845 |
2,801,709 |
| GreenLight Rezum PLASMA System UroLift |
80,184,845 |
68,095,210 |
12,089,636 |
| XprESS |
21,752,425 |
16,221,856 |
5,530,569 |
| Thopaz |
57,798,050 |
48,183,361 |
9,614,689 |
| Spectra Optia |
27,156,262 |
22,036,283 |
5,119,980 |
|
Total |
498,477,099 |
454,029,916 |
44,447,183 |
AposHealth
1. The potential cost savings in the first year of treatment for 100 patients that are prescribed AposHealth is £245,000, and the cumulative savings over a period of 5 years for those 100 patients is £509,600, according to NICE resource impact assessment.
Annex 3: A practical guide to MTFM payment mechanisms
Background
2. The MTFM Policy team have sought feedback from the Health Innovation Network, and ICSs on the practicalities of implementing the policy. This has generated substantial learning on the challenges systems face in establishing how supported technologies can be funded (and their benefits realised).
3. In response to this feedback, this annex provides a practical guide to support providers, commissioners and other key policy stakeholders in seeking solutions when discussing how to fund MTFM supported technologies in different funding scenarios.
4. The NHS Payment Scheme recommends use of blended payments, which is a NHS Long Term Plan commitment (see page 101). On this basis, the MTFM requests providers and commissioners together explore how supported technologies can be funded and savings can be realised.
5. In previous years, feedback from the Health Innovation Network to the central policy team has suggested that, particularly where ICS relationships are still maturing, alternative funding arrangements have been agreed. This notably includes requests that providers fund new technology implementations from their pre-existing fixed payment allocations.
6. Questions about how best to identify funding have posed a barrier to MTFM supported technology adoption, particularly where systems are in deficit. NHS England has received feedback that some providers have been asked to find funding from within their fixed payments, with no scope for pass through payments to cover technology costs, and no inclusion of MTFM technology-related implementation costs.
7. While we are providing guidance to support navigation of the scenarios outlined in paragraph 18 onwards, the MTFM Policy team will be working with local health innovation networks, and the NHS England pricing team to understand how ICBs are moving towards the advocated payment mechanisms.
ICS duty to adopt innovation
8. Where the MTFM financial mechanisms, which are designed to incentivise innovation, are not being used, the more rigid funding arrangements outlined above have been found to hinder adoption of supported technologies.
9. Failing to adopt supported technologies where clinically appropriate contravenes the NHS Standard Contract (section 2.2) and NHS Payment Scheme guidance. ICBs have a duty in exercising their functions to promote innovation in the provision of health services, as described in section 13Z39 of the NHS Act 2006 (as superseded by section 25 of the Health and Care Act 2022). Agreeing payment with providers is part of their functions, so the legal duty to promote innovation applies, and innovation must be accounted for in payment arrangements (that is, agreeing their aligned payment and incentive arrangement). Pages 13, 15 and 18 of NHS England’s integrated care systems design framework provide more detail on this duty.
Funding provision
10. The aligned payment and incentive blended payment model is made up of a fixed payment and a variable payment (for example, for elective care). Provider/commissioner relationships with an expected annual value below £0.5 million are subject to low volume activity payment arrangements. The costs of MTFM supported technologies are excluded from both these payments. Commissioners should instead reimburse providers for MTFM technology costs via ‘pass through payments’ based on the cost and volume of technology use.
11. The fixed payment between commissioners and providers should also include an amount for any implementation costs not covered by ‘pass through payments’. Providers and commissioners should submit a variation to NHS England, using the required template, if they are not following this guidance.
12. Ideally, these arrangements should be agreed ahead of the start of each financial year so that fixed payments are set appropriately, and the commissioner can identify the funding required in year for pass through payments. If a provider has signed up to a fixed payment without these considerations, then it needs to identify how it will meet any funding requirements. If the commissioner is not reimbursing the innovative products in accordance with the NHS Payment Scheme, then it should submit a variation to NHS England, along with justification, using the required template.
13. Providers and commissioners should identify where necessary funding that could be made available; through using funding kept in reserve, or exploring whether any capacity benefit released from adopting MTFM supported technologies could be used to support use of the Elective Recovery Fund. If these two options are not possible, providers and commissioners should establish arrangements to ensure payment guidance is followed next year (2025/26) and revise service delivery plans to account for the effects of using the MTFM technologies. Providers and commissioners should work together to ensure financial risk is shared, especially when costs and benefits may sit in different parts of the system.
Next steps for health innovation networks
14. This annex explains the expectation under the NHS Payment Scheme and MTFM policy that ICBs identify additional monies to fund MTFM technologies regardless of historical MTFM-related funding arrangements.
15. Health innovation networks encountering this issue should share the narrative with their ICB stakeholders, together with this annex, to aid funding and commissioning conversations.
Existing adopters of technologies
16. Some providers may have already adopted MTFM supported technologies. If this is the case, MTFM funding mechanisms should only be used if adoption has since ceased and implementation funding is required to reintroduce the technologies in the services offered, or additional funding is required to offer increased/improved patient access.
17. Implementation costs associated with adopting MTFM supported technologies or increasing their use should be incorporated in the fixed payment, with technology costs forming part of the variable element (as ‘pass through payments’ based on technology cost and volume). If MTFM supported technology costs are included in the fixed payment, they should be removed and replaced with ‘pass through payments’ based on the cost and volume of MTFM supported technology use.
Scenarios where MTFM funding mechanisms can be used
18. The 2 scenarios where MTFM funding mechanisms can be used are:
- a provider wants to implement an MTFM supported technology for the first time
- a provider wants to increase use of an MTFM supported technology that it has already adopted
Scenario A: A provider wants to implement an MTFM supported technology for the first time
- identify implementation costs associated with introducing the technologies, in consultation with clinical and non-clinical colleagues with Health Innovation Network support
- understand how using the technology will release capacity and what setting/case mix of patients will be treated with this (released) capacity
- commissioners pay for the relevant MedTech product(s) via ‘pass through payments’ based on anticipated cost and volume
- commissioner and provider agree a timeline for when the technology will be incorporated in BAU and become part of the fixed payment, and ‘pass through payments’ will no longer be needed
Implementing technologies for the first time
1. MedTech product costs not included in provider costs
2. Identify implementation costs
3. Commissioners pay for MedTech product costs based on anticipated cost and volume
4. Supplementary cost of implementation included in fixed payment
5. Provider and commissioner agree timeline for incorporating MedTech in BAU
Scenario B: A provider wants to increase usage of an MTFM supported technology that it has already adopted
- agree any additional implementation costs with commissioner and anticipate the cost and volume of increased technology usage
- understand how using the technology will release capacity and what setting/case mix of patients will be treated with this (released) capacity
- commissioners pay for the relevant MedTech product(s) by either:
- agreeing further ‘pass through payment’ based on anticipated cost and volume (and maintaining any pre-existing MTFM-related payments in the fixed element)
- stripping any historical payment out of the fixed element and paying for all MTFM-related technology costs via ‘pass through payments’ (to simplify funding flows)
- commissioner and provider agree a timeline for when the relevant technologies will be incorporated in BAU and become part of the ‘pass through payment’, and ‘pass through payments’ will no longer be needed
Increasing usage of already adopted technologies
1. Some MedTech product costs included in provider costs
2. Understand how additional technology use will release capacity and how that will be used
3. Commissioners pay for MedTech product costs based on anticipated cost and volume
4. Supplementary cost of implementation included in fixed payment
5. Provider and commissioner agree timeline for incorporating MedTech in BAU
Annex 4: Support offered for each technology
1. Technologies supported by the MTFM will receive support, the level of which will depend on how long they have been supported by the policy and the extent of their adoption and spread across the healthcare system. The support in each of the 3 phases of adoption is as follows:
Phase 1: Onboarding
2. Prepare and align the MTFM policy team, Health Innovation Network, supplier and other stakeholders, and plan a 2-year programme of support.
3. MTFM Policy team support:
- work with lead clinicians and GIRFT to endorse the national spread and adoption of selected technologies
- signal to the system that the technology will be supported and promote the benefits to the system, clinicians and patients
- complete a memorandum of understanding with technology supplier to establish data sharing principles and procedures
- work with NHS Supply Chain to facilitate the inclusion of the technology on a procurement framework
- facilitate a series of partnership learning sessions to build relationships and ensure technology suppliers understand the policy mechanisms and processes
- undertake national communications work to promote the new technologies
- produce and publish the MTFM policy guidance document
- ensure the NHS Payment System documentation is updated with new technologies, and any changes to mechanisms are understood
4. Health Innovation Network support:
- Health Innovation Network National Programme Director appoints dedicated national Health Innovation Network Product Lead for each technology to help co-ordinate and drive spread and adoption
- understand baseline adoption data
- agree a ‘typical’ 2-year programme of support (spread and adoption plan), subject to understanding the level of pathway transformation required in line with previous experience
- educate the wider Health Innovation Network about the technologies and NICE guidance
- work with the MTFM Policy team to develop communications for the sector
- create working groups where needed
Phase 2: Adoption and spread
5. Focus on driving adoption for each supported technology at scale.
6. MTFM Policy team support:
- drive the policy by working with national clinical leads, ICSs, MTFM champions and regional NHS colleagues
- feedback real-world learning and evidence to NICE
- support the resolution of policy compliance issues escalated by Health Innovation Networks
- support the communication of good news stories
- offer targeted support from data intelligence
7. Health Innovation Network support:
- Health Innovation Network adoption and spread support starts for the new technology (likely to be 1 April). This work is led by the HeaIth Innovation Network National Programme Director and Health Innovation Network Technology Lead, and is delivered by each of the 15 health innovation networks. Drive the spread and adoption plan in local healthcare providers
- support providers to develop their case for change
- support business case development and the commissioning agreement process
- support the implementation process and celebrate successes
- complete the quarterly assurance reporting tool (QART)
- raise compliance issues with the MTFM Policy team
- facilitate round table discussions to overcome barriers to adoption
Phase 3: Graduation
8. Review the success of the spread and adoption phase, ensure adoption is sustained and any ongoing business cases are supported to completion, and preserve knowledge and tools for future work.
9. MTFM policy team support:
- communicate success of the technology to the sector
- clearly signal guidance and tools for further adoption by sector
- clearly signal relevant contacts and information/NHS Futures (central inbox)
- collate learnings and reflections to inform future programmes in final project report
- understand and signal any available future support from other programmes for the technologies
- continue monitoring adoption via NHS Supply Chain reporting
- continue to support organisations that want to implement the technology by linking them in with partners that can support the process
- ensure correct tariffs/funding mechanism is in place going forward
10. Health Innovation Network support:
11. When a technology reaches the graduation phase, the Health Innovation Network will no longer be formally required to provide implementation support for a technology.
12. Health Innovation Network National Programme Director and/or Health Innovation Network Technology Lead to co-ordinate Health Innovation Network colleagues to:
- continue to support business cases that are in progress
- complete implementation projects that have begun
- complete patient stories/business cases to help future implementation projects
- contribute to the final project report
- contribute to learnings and reflections
Post graduation
11. The MTFM Policy team can direct any providers wanting to implement a technology to guidance, tools and other policy partners that can help.
Phase of support for MTFM supported technologies in 2024/25
| Phase as at 1 April 2024 | Technology | Cohort | Due to graduate |
|---|---|---|---|
|
2 |
AposHealth |
2024/25 |
April 2027 |
|
2 |
Spectra Optia |
2022/23 | April 2025 Due to complexity of Phase 1, Health Innovation Network support will continue phase 2 until March 2025 |
|
2 |
UroLift |
2022/23 |
April 2025 |
|
2 |
GreenLight |
2022/23 |
April 2025 |
|
2 |
Rezum |
2022/23 |
April 2025 |
|
2 |
PLASMA System |
2022/23 |
April 2025 |
|
2 | XprESS |
2022/23 |
August 2024 |
|
2 | Thopaz+ |
2022/23 |
August 2024 |
|
Graduated |
HeartFlow |
2021/22 |
April 2024 |
|
Graduated |
SecurAcath |
2021/22 |
April 2024 |
|
Graduated3 |
gammaCore |
2021/22 |
April 2024 |
|
Graduated3 |
PLGF |
2021/22 |
April 2024 |
Publication reference number: PRN01155