Background
NHS England’s Newborn Hearing Screening Programme (NHSP) completed an analysis of data for every baby born in England from 2018-2023. This identified 4 trusts, covering 5 services, that reported significantly fewer cases of permanent childhood hearing impairment than expected. A thorough investigation of these services identified systemic issues, including poor-quality practices, inadequate staff training, substandard data and report management, inconsistencies in care, ineffective peer review processes, and a lack of UK Accreditation Service (UKAS) Improving Quality in Physiological Services (IQIPS) accreditation. In response to these findings, national recommendations were issued to integrated care boards (ICBs) to assess compliance with established standards and best practices. Widespread non-compliance confirmed these issues were systemic rather than isolated. This led to the development of the Paediatric Hearing Services Improvement Programme, created in collaboration with service providers, ICBs, NHS England regions, the Care Quality Commission, multidisciplinary experts, professional bodies, and patient groups. The Programme’s primary focus is to conduct a nationally coordinated review of all paediatric audiology services within the NHS in England and aims to identify and recall babies and children at risk, mitigate harm caused by misdiagnosis or delayed diagnosis, and support services in delivering quality improvement interventions.
Purpose
The operational guidance aims to support NHS England regions, integrated care boards, and NHS trusts to conduct patient recalls, identifying babies and children at risk, and address harm caused by misdiagnosis or delayed diagnosis. It has been informed by reviews of the initial 5 services. To ensure continued improvement, please send suggestions that could improve this operational guidance to england.csohearingprogramme@nhs.net.
Guiding principles
In line with the National Patient Recall Framework, the following principles guide the national review and recall process for paediatric audiology services across England.
- The recall and reassessment process must be driven by patient safety, ensuring that risks are minimised and any harm is mitigated.
- The needs of the child and family must be central to all recall processes. This includes providing clear communication, support, and ensuring that the patient’s voice is heard throughout the process.
- Open, honest communication with patients, carers, and healthcare professionals is essential. Any concerns about care should be acknowledged, and apologies provided when appropriate.
- The review and recall process should be coordinated across different healthcare providers, ensuring a unified response that minimises variation and supports continuous improvement.
- Recognising the stress recalls may cause, families and staff should have access to emotional and psychological support, as well as assistance with transport, language, or accessibility needs.
- Learning from the outcomes of the review and recall process will inform future practice and improve service quality across regions.
National review and recall process – stages of assurance review
The national review and recall process is supported by a national service assurance framework (figure 1 – see below) designed to risk-stratify services. The primary objective of this framework is to identify babies and children who may be at risk of harm due to missed or incorrect diagnoses of hearing loss. Through systematically evaluating services, the framework ensures that those with higher risks receive priority attention, enabling interventions to mitigate potential harm and improve patient outcomes.
Figure 1: National service assurance framework – stages 1 to 4
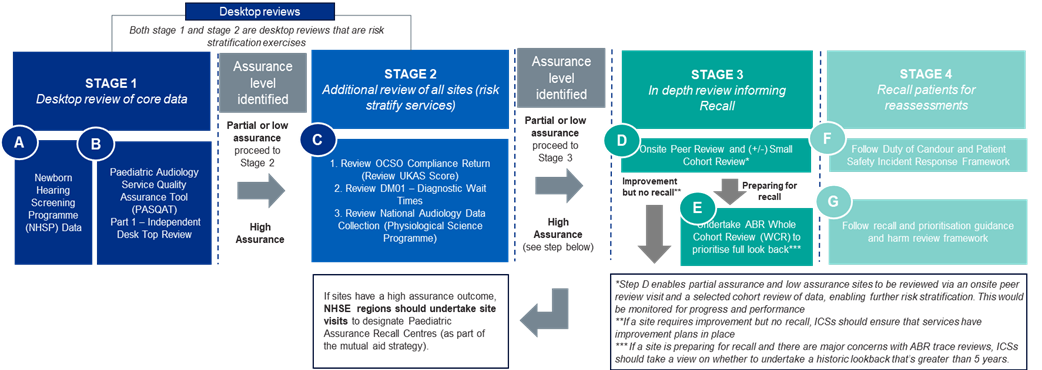
Stage 1 of the National service assurance framework: desktop review of core data (lead: NHS England regions)
This stage involves a comprehensive desktop review to assign an initial assurance level rating for each service. The review uses two key data tools: the Newborn Hearing Screening Programme (NHSP) data (A) and offsite independent desktop assessment (B). Services are rated as having an assurance level of high, partial or low, before moving to stage 2 for further analysis.
Stage 2 of the National service assurance framework: additional review and risk stratification (lead: NHS England regions)
Stage 2 uses 3 data tools: the UKAS IQIPS score status, diagnostics waiting times and activity (DM01) data (covering both adult and paediatric audiology, with disaggregation planned), and data from the National Audiology Data Collection as part of the National Physiological Science Transformation Programme. Once the data from these tools is analysed, an assurance outcome for each service is confirmed. There are two outcomes.
- High assurance outcome (lead: NHS England regions)
For services that achieve a high assurance rating, NHS England regions should conduct site visits to confirm and designate these services as paediatric assurance recall centres, as part of the mutual aid strategy. A service is defined as high assurance if it is UKAS accredited with no data concerns identified. - Low or partial assurance outcome (lead: ICBs)
If services receive low or partial assurance ratings, they will progress to stage 3 for an in-depth review. A partial assurance service has minor data concerns, a low assurance service has major data concerns.
Stage 1 and 2 of the national service assurance framework has been completed for all 140 paediatric audiology services in England. This element of the framework will only need to be followed if new services are identified.
Stage 3 of the National service assurance framework: in-depth review to inform patient recall (lead: ICBs)
The purpose of stage 3 is to determine whether a full cohort review and recall of patients is necessary. During this stage, onsite peer review visits are led by the ICB and supported by subject matter experts (SMEs). These visits will be prioritised based on the risk stratification exercise (stage 1 and 2). Alongside the onsite peer review, an auditory brainstem response (ABR) small cohort review should be conducted. In cases where there are significant concerns with the ABR service, or a full cohort review is expected, the ABR small cohort review may be bypassed, and the service can proceed directly to a full cohort review. Following the onsite review and ABR small cohort review there will be 2 outcomes.
- Improvement but not recall: For sites that require improvement but do not need to recall patients, it is essential that ICBs ensure these services develop and implement comprehensive improvement plans, with reporting through relevant governance forums. This proactive measure allows for ongoing service improvements addressing key areas of concern.
- Preparing for recall: If a site is preparing for recall they should follow the full cohort review guidance. If there are major concerns with ABR trace reviews, ICBs should take a view on whether to undertake a review of more than 5 years.
Onsite review
The onsite visit provides independent and external assessment of the safety of the service, with review of service documentation related to clinical governance. This should include clinical protocols, audit, effective waiting list management, safe staffing levels, registration status of staff, appraisals, education and training. Prior to the visit the paediatric audiology department should make the following available to the assessing team:
- evidence of staff registration, including a list of names and registration numbers for appropriate clinical staff
- current waiting times for: assessment (new and follow up), paediatric hearing aid fitting, paediatric hearing aid review, paediatric hearing aid repair and mould appointments
- triage protocol and standard operating procedures
- assessment/management protocols
- clinical pathways or guidelines for paediatric audiology (including referral methods, appointment lengths, staff establishment and qualifications)
- 5 year yield report (from the NHSP team)
The review team will provide:
- findings and recommendations from stage 1 and 2
- NHSP data for the site which includes detail of NHSP yield for immediate referral and following a screen pass, mean and median days to confirmation of hearing loss
- number of cases not determined at their first and last visit to audiology
- detail of any other reviews that may be underway. For example, if there were concerns regarding the quality of an ABR, a separate audit of a small cohort may also be underway. This should not delay an on-site peer review, but the reviewing team should be made aware of this. See annex 1: Onsite review information
At the end of the visit verbal feedback will be provided to the service. A written report will be provided to the trust within an agreed timeframe outlining the findings and recommendations. This will be shared with the ICB Strategic Response Team to enable further actions to be addressed.
Auditory brainstem response small cohort review
An auditory brainstem response (ABR) small cohort review framework is available to support this step. Where it is shown that an ABR small cohort review would confirm the need for a full cohort review, the service may move directly to a full cohort review. The review is conducted using random selection to create a non-biased assessment of ABR testing and case management. Typically, a 5-year period is reviewed, although this can be extended in cases of serious incidents, whistleblowing, parental complaints, disputed ABR peer review findings, or other relevant local information.
Steps for an ABR small cohort review.
- Use the ABR small cohort review framework and template to conduct the review.
- Communicate with the trust to agree on data sharing requirements, ensuring all data is anonymised.
- Request cohort data from the NHSP team.
- Identify SMEs to conduct the review sourced from the national SME register.
- Share the outcome and recommendations with the relevant trust and ICB.
Any patients requiring recall as part of the ABR small cohort review should be notified to the service immediately, and the recall should be conducted without delay.
Full cohort review guidance
If the results of the onsite peer review or ABR small cohort review suggest that a full cohort review is necessary, the service should proceed with reviewing ABR traces over the past 5 years. This includes all cases referred via the NHSP and other referrals identified through local trust data. Typically, a 5-year period is reviewed, although this can be extended in cases of serious incidents, whistleblowing, parental complaints, disputed ABR peer review findings, or other relevant local information.
Steps for a full cohort review.
- Use the provided framework and reporting templates detailed in the further technical guidance.
- Prioritise full cohort reviews based on service needs.
- Communicate with the trust regarding data sharing, ensuring anonymisation of all data.
- Request cohort data from the NHSP team.
- Identify SMEs to conduct the review, using the national SME register.
- Share the review outcome and recommendations with relevant governance forums
Following the completion of a full cohort review, the following actions should be taken.
- Compile a list of patients requiring recall and reassessment, prioritised based on risk factors.
- Provide feedback to relevant governance forums.
- Consider immediate mitigations, including partial or full closure of services.
- Implement the Patient Safety Incident Response Framework for incident management.
- Develop an urgent service development plan to address identified risks, which may include staff training, onsite or remote aid, and equipment replacement.
- Work with regions to identify mutual aid from designated paediatric assurance recall centres.
- Proceed to stage 4 – recall and reassessment.
Stage 4 of the National service assurance framework – recall and reassessment (lead: ICBs)
The goal of stage 4 is to recall and reassess patients to determine if harm has occurred. Patients should be prioritised for reassessment based on the details within the further technical guidance, and the Duty of Candour must be followed. Mutual aid should be identified to support the reassessment process, and a harm review must be completed after reassessment using the Learn from Patient Safety Events Framework. The results of the review should be reported to the relevant governance forums.
Distraction testing
If a service has been discharging patients based solely on distraction test results, which does not meet discharge criteria, a full recall of all patients over the past 5 years is required.
Guidance for visual reinforcement audiometry only services
Onsite peer visits for visual reinforcement audiometry (VRA)-only services should assess quality and safety, focusing on:
- identifying issues through submitted photographs and information
- observing VRA testing for 2 patients per site during a site visit
- reviewing a sample of cases from the permanent childhood hearing impairment register for children aged 5-6 years old to compare VRA results with later pure tone audiometry testing
If no issues are identified, an action plan should be created for required improvements. However, if concerns arise, an incident should be declared, and a full cohort review may be necessary.
Following and implementing the service assurance framework: system roles and responsibilities
|
System level |
System roles and responsibilities |
|
Integrated care boards (ICBs) |
|
|
NHS England regions |
|
|
NHS England national |
|
Waiting times and mutual aid
All paediatric audiology services are required to report waiting times through the diagnostics waiting times and activity (DM01). Priority must be given to cases that require clinical assessment, particularly those involving the review, recall, and reassessment of children in services identified for a full cohort review. These cases should take precedence over standard waiting list management to ensure timely care for children at risk. Guidance on provider collaboratives and Getting It Right First Time guidance can be used to explore opportunities for mutual aid within their system. Paediatric assurance recall centres offering mutual aid should prioritise supporting other services involved in reviewing and reassessing children, ensuring that the most urgent cases are addressed efficiently. For sites providing mutual aid, that hold paediatric audiology UKAS IQIPS accreditation, UKAS should be notified of the additional work pressure and expected impact to waiting times.
Data
All integrated care boards are required to report data on service assurance status, as well as the status of reassessment, review, and recall processes. This data must be submitted through the regional team to the national programme as part of a monthly data collection. The reporting process will be coordinated and cascaded through the national and regional operational systems, ensuring timely completion and return of data to support the ongoing monitoring and improvement of paediatric audiology services.
Quality standards
All services should be working to the most recent published standards and guidelines. UKAS IQIPS is the only national accreditation body for paediatric audiology services. It is the recommended quality assurance scheme for hearing services. All services should achieve, be working towards or maintain accreditation.
It is best practice that practicing audiologists should be registered with a professional registration body. There are two registration bodies in the United Kingdom for audiologists: Academy for Health Care Science (voluntary) or Health and Care Professions Council (statutory). All audiologists should be working towards the standards of Good scientific practice and maintaining their clinical competencies through regular CPD and follow current audiology clinical guidelines.
National register for subject matter experts
A subject matter experts (SME) register has been established to support regions and ICBs in carrying out necessary actions for service improvement and patient care. These SMEs will provide external, independent reviews of services, assist with the in-depth review, recall, and reassessment of patients in identified services, and offer mutual aid where required. Given the limited national pool of SMEs in paediatric audiology, the register ensures that expertise can be accessed across ICBs and regions, particularly in areas where services are more challenged in meeting the programme’s goals. The rationale for this initiative stems from the need to enable cross-region and cross-ICB collaboration, allowing for efficient support through a national SME register that includes remuneration for staff time. All professionals listed on the register have undergone competency assessments, voluntarily joined with the support of their line managers, and will negotiate duties in addition to their substantive roles. SMEs involved in service reviews must be registered nationally, with local support being sought first and national resources accessed as needed. SMEs from the register will support peer review visits for services with low or partial assurance status, conduct small and full cohort reviews, participate in physical reassessments for recalled patients, double-report auditory brainstem response traces for at-risk services, and provide ad-hoc advice on service improvement actions.
The national SME register offers several benefits to ICBs and regions, including access to a pool of competency-checked professionals, a dedicated inbox for SME requests, and a streamlined process for matching SMEs based on availability, competency, and location. This flexible system allows for requests to be fulfilled across regions, significantly expanding capacity and reducing delays. SMEs may work remotely when needed, and a network for knowledge sharing will be fostered. ICBs will work closely with SMEs to determine the time required for tasks and confirm payment based on hours worked. The national SME register provides a single point of contact through a dedicated inbox at england.csohearingprogramme@nhs.net, where ICBs can send requests for SME capacity. This ensures streamlined communication and efficient coordination for accessing SME support.
Governance
The national governance structure (Figure 2) supports the delivery of the review and recall process at speed, ensures efficient and responsive local implementation, whilst providing clear escalation routes to allow for effective national oversight.
At integrated care board level
A strategic response group should be established in locations where services have low or partial assurance, as identified in stage 1 of the national service assurance framework. The group is responsible for identifying a team to drive the delivery of the national framework for review and recall, including securing necessary resources. It will collaborate with national and regional colleagues to use the SME register when needed, ensure compliance with regular reporting, and escalate risks and issues for regional mitigation. In cases where services do not require recall but need improvement, the integrated care board (ICB) strategic response group will ensure improvement plans are in place to uphold high standards and quality of care. The ICB strategic response group will formally report to the Regional Audiology Oversight Group, ensuring coordinated efforts to improve service quality, mitigate levels of harm, and enhance patient outcomes.
Figure 2: governance structure at integrated care board, regional and national level
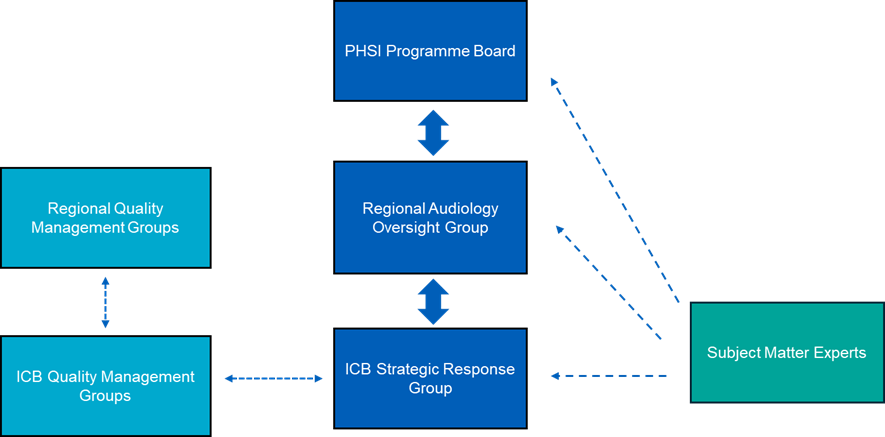
At NHS England regional level
Regions should establish a regional audiology oversight group including relevant representation from the region in conjunction with ICBs. Key responsibilities include:
- oversight and assurance of the review and recall process, and ensuring ICBs comply with the national data collection and reporting processes
- determining regional patient reassessment locations (as outlined on the national service assurance framework)
- driving service and quality improvement across the region, ensuring services that do not require recall, but instead broader improvements, have local plans in place
- escalating risks and issues for mitigation, particularly those requiring national coordination)
- formally providing reports to relevant quality governance forums and to the National Paediatric Hearing Services Improvement Programme Board.
- providing oversight and assurance, coordinating mutual aid and capacity as required. The group will escalate risks and issues to the national programme board
At NHS England national level
The National Paediatric Hearing Services Improvement Programme Board will provide strategic direction and national guidance.
Further technical guidance
Further detailed and technical guidance is available to integrated care boards and subject matter experts including:
- auditory brainstem response small cohort review framework and template (for use during stage 3)
- on-site review template, agenda for visit, notes for reviewers, outcome and feedback template (for use during stage 3)
- full cohort review framework and template (for use during stage 3)
- recall, prioritisation and harm review framework (for use during stage 4)
- example clinical testing mitigation (for use during stage 4)
- triage criteria for paediatric audiology referrals and reviews (for use during stage 4)
Annex 1: Onsite review information
Domain: clinical governance
|
Requirement |
Information/examples |
|
Clinical document management process |
Document control policy or from discussion with service lead |
|
Examples of clinical audit |
Audit calendar and examples of a minimum of 2 audits into clinical processes |
|
Evidence of appraisal process |
Examples of a minimum of 2 appraisal records, including personal development plan |
|
Waiting list management, including triage |
Standard operating procedures, verbal discussion with head of service relating to performance metrics |
Domain: equipment and clinical environments
|
Equipment register |
Register with evidence of calibration due dates, calibration certificates, business continuity relating to equipment |
|
Maintenance and calibration |
Equipment in clinical rooms viewed during clinical observations |
|
Room configurations |
Rooms observed during clinical observations |
Domain: staffing
|
Embedded clinical pathways |
SME will need to observe 2x assessments for patients aged 8 month to 2 years (one must be suitable for visual reinforcement audiometry 2x assessments for patients aged 3 to 5 years SME will need to perform a case note review, with access to patient management system for 1x recent permanent childhood hearing impairment hearing aid issue (full record from screen to latest appointment should be reviewed) 1x school aged hearing aid review (full record from screen to latest appointment should be reviewed) |
|
Incident and risk reporting |
Risk register for department available |
|
Semi-structured staff interviews – template detailed in the further technical guidance. |
Departmental staff to be made available between 10am and 3pm |
Publication reference: PRN01584_ii

